Chemistry:Osmium compounds
| Oxidation states of osmium | |
|---|---|
| −2 | Na2[Os(CO)4] |
| −1 | Na2[Os4(CO)13] |
| 0 | Os3(CO)12 |
| +1 | OsI |
| +2 | OsI2 |
| +3 | OsBr3 |
| +4 | OsO2, OsCl4 |
| +5 | OsF5 |
| +6 | OsF6 |
| +7 | OsOF5 |
| +8 | OsO4, Os(NCH3)4 |
Osmium compounds are compounds containing the element osmium (Os). Osmium forms compounds with oxidation states ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9[1] and is encountered only in xenon,[2][3] ruthenium,[4] hassium,[5] iridium,[6] and plutonium.[7][8] The oxidation states −1 and −2 represented by the two reactive compounds Na2[Os4(CO)13] and Na2[Os(CO)4] are used in the synthesis of osmium cluster compounds.[9][10]
Oxides
Osmium tetroxide is the most notable compound of osmium, having many uses. The name "osmium" even derives from Greek "Ancient Greek:" because of the smell of osmium tetroxide.[12] It also has a number of unusual properties, one being that the solid is volatile. Its volatility, along with its strong oxidizing power, is the origin of its quite serious toxicity - inhalation provides a very effective route for the compound to react with tissue. The compound is colourless, but most samples appear yellow.[13] This is most likely due to the presence of the impurity OsO2, which is yellow-brown in colour.[14] In biology, its property of binding to lipids has made it a widely used stain in electron microscopy. OsO4 is formed slowly when osmium powder reacts with O2 at ambient temperature. Reaction of bulk solid requires heating to 400 °C.[15]
- [math]\ce{ Os + 2O2 ->[\Delta T] OsO4 }[/math]
OsO4 is a Lewis acid and a mild oxidant. It reacts with alkaline aqueous solution to give the perosmate anion OsO4(OH)2−2.[16] This species is easily reduced to osmate anion, OsO2(OH)2−4. When the Lewis base is an amine, adducts are also formed. Thus OsO4 can be stored in the form of osmeth, in which OsO4 is complexed with hexamine. Osmeth can be dissolved in tetrahydrofuran (THF) and diluted in an aqueous buffer solution to make a dilute (0.25%) working solution of OsO4.[17] With tert-BuNH2, the imido derivative is produced:
- OsO4 + Me3CNH2 → OsO3(NCMe3) + H2O
Similarly, with NH3 one obtains the nitrido complex:
- OsO4 + NH3 + KOH → K[Os(N)O3] + 2 H2O
The [Os(N)O3]− anion is isoelectronic and isostructural with OsO4. OsO4 is very soluble in tert-butyl alcohol. In solution, it is readily reduced by hydrogen to osmium metal. The suspended osmium metal can be used to catalyze hydrogenation of a wide variety of organic chemicals containing double or triple bonds.
- OsO4 + 4 H2 → Os + 4 H2O
OsO4 undergoes "reductive carbonylation" with carbon monoxide in methanol at 400 K and 200 sbar to produce the triangular cluster Os3(CO)12:
- 3 OsO4 + 24 CO → Os3(CO)12 + 12 CO2[15]
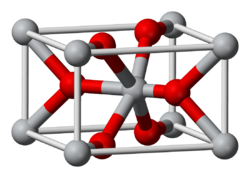
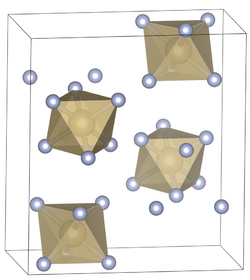
Osmium dioxide is another known oxide of osmium, which can be obtained by the reaction of osmium with a variety of oxidizing agents, including, sodium chlorate, osmium tetroxide, and nitric oxide at about 600 °C.[18][19] It does not dissolve in water, but is attacked by dilute hydrochloric acid.[20][21] The crystals have rutile structure.[22] Unlike osmium tetroxide, OsO2 is not toxic.[23]
Halides
Fluorides
Osmium hexafluoride is one of the 17 known binary hexafluorides, which can be made by the direct reaction of osmium metal exposed to an excess of elemental fluorine gas at 300 °C. It is a yellow crystalline solid that melts at 33.4 °C and boils at 47.5 °C.[24] The solid structure measured at −140 °C is orthorhombic space group Pnma. Lattice parameters are a = 9.387 Å, b = 8.543 Å, and c = 4.944 Å. There are four formula units (in this case, discrete molecules) per unit cell, giving a density of 5.09 g·cm−3.[25] The OsF6 molecule itself (the form important for the liquid or gas phase) has octahedral molecular geometry, which has point group (Oh). The Os–F bond length is 1.827 Å.[25] Partial hydrolysis of OsF6 produces OsOF4.[26] Osmium pentafluoride is a tetramer in the solid state that can be prepared by reduction of osmium hexafluoride with iodine as a solution in iodine pentafluoride:[27]
- 10 OsF6 + I2 → 10 OsF5 + 2 IF5
Chlorides
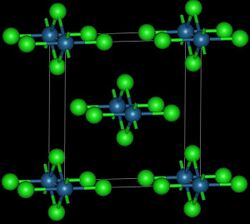
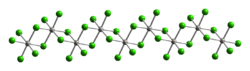
Osmium tetrachloride exists in two crystalline forms, and is used to prepare other osmium complexes. It was first reported in 1909 as the product of chlorination of osmium metal.[28] This route affords the high temperature polymorph:[29]
- Os + 2 Cl2 → OsCl4
This reddish-black polymorph is orthorhombic and adopts a structure in which osmium centres are octahedrally coordinated, sharing opposite edges of the OsCl6 octahedra to form a chain.[30] A brown, apparently cubic polymorph forms upon reduction of osmium tetroxide with thionyl chloride:[31]
- OsO4 + 4 SOCl2 → OsCl4 + 2 Cl2 + 4 SO2
Osmium tetroxide dissolves in hydrochloric acid to give the hexachloroosmate anion:
- OsO4 + 10 HCl → H2OsCl6 + 2 Cl2 + 4 H2O
Bromides
Osmium tetrabromide is a black solid that can be produced by heating osmium tetrachloride and bromine under pressure. As determined by X-ray crystallography, osmium tetrabromide is an inorganic polymer. It is isomorphous with platinum tetrabromide and technetium tetrachloride. As such, osmium is in octahedral coordination. Each osmium center bonds to four doubly bridging bromide ligands and two mutually cis terminal bromide ligands.[32] Osmium tribromide, OsBr3, is the only other binary osmium bromide is that has been crystallized.[33]
Iodides
Osmium(I) iodide is a metallic grey solid produced by the reaction of osmium tetroxide and hydroiodic acid heated in a water bath for 48 hours in a carbon dioxide atmosphere. It is an amorphous compound.[34] Osmium(II) iodide is a black solid[35] produced by the reaction of osmium tetroxide and hydroiodic acid at 250 °C in nitrogen:[34]
- OsO4 + HI → OsI2 + H2O
This compound decomposes in contact with water.[35] Osmium(III) iodide is a black solid that is produced by heating hexaiodoosmic acid (H2OsI6).[34] This compound is insoluble in water.[35] Osmium(IV) iodide has been claimed to exist, although the supposed way to prepare it (reacting osmic acid, H4OsO6, with hydroiodic acid[36]) produced dihydroxonium hexaiodoosmate instead of the tetraiodo compound, and instead contained mono, di and tri-iodo osmium compounds.[34]
Borides
Osmium borides are notable for their potentially high hardness. It is thought that a combination of high electron density of osmium with the strength of boron-osmium covalent bonds will make osmium borides superhard materials, however this has not been demonstrated yet. For example, OsB2 is hard (hardness comparable to that of sapphire), but not superhard.[37] These borides are produced in vacuum or inert atmosphere to prevent formation of osmium tetroxide, which is a hazardous compound. Synthesis occurs at high temperatures (~1000 °C) from a mixture of MgB2 and OsCl3.[37] Three osmium borides are known: OsB, Os2B3 and OsB2. The first two have hexagonal structure,[38] similar to that of rhenium diboride. Osmium diboride was first also sought as hexagonal,[39] but one of its phases was later reassigned to orthorhombic.[37][40] In recent methods of synthesis, it has also been found that a hexagonal phase of OsB2 exists with a similar structure to ReB2.[41]
See also
References
- ↑ Stoye, Emma (23 October 2014). "Iridium forms compound in +9 oxidation state". Chemistry World. Royal Society of Chemistry. http://www.rsc.org/chemistryworld/2014/10/iridium-oxide-cation-oxidation-state-9.
- ↑ Selig, H.; Claassen, H. H.; Chernick, C. L.; Malm, J. G. et al. (1964). "Xenon tetroxide – Preparation + Some Properties". Science 143 (3612): 1322–1323. doi:10.1126/science.143.3612.1322. PMID 17799234. Bibcode: 1964Sci...143.1322S.
- ↑ Huston, J. L.; Studier, M. H.; Sloth, E. N. (1964). "Xenon tetroxide – Mass Spectrum". Science 143 (3611): 1162–1163. doi:10.1126/science.143.3611.1161-a. PMID 17833897. Bibcode: 1964Sci...143.1161H.
- ↑ Barnard, C. F. J. (2004). "Oxidation States of Ruthenium and Osmium". Platinum Metals Review 48 (4): 157. doi:10.1595/147106704X10801.
- ↑ "Chemistry of Hassium". Gesellschaft für Schwerionenforschung mbH. 2002. http://www.gsi.de/documents/DOC-2003-Jun-29-2.pdf.
- ↑ Gong, Yu; Zhou, Mingfei; Kaupp, Martin; Riedel, Sebastian (2009). "Formation and Characterization of the Iridium Tetroxide Molecule with Iridium in the Oxidation State +VIII". Angewandte Chemie International Edition 48 (42): 7879–7883. doi:10.1002/anie.200902733. PMID 19593837.[|permanent dead link|dead link}}]
- ↑ Kiselev, Yu. M.; Nikonov, M. V.; Dolzhenko, V. D.; Ermilov, A. Yu.; Tananaev, I. G.; Myasoedov, B. F. (17 January 2014). "On existence and properties of plutonium(VIII) derivatives". Radiochimica Acta 102 (3): 227–237. doi:10.1515/ract-2014-2146.
- ↑ Zaitsevskii, Andréi; Mosyagin, Nikolai S.; Titov, Anatoly V.; Kiselev, Yuri M. (21 July 2013). "Relativistic density functional theory modeling of plutonium and americium higher oxide molecules". The Journal of Chemical Physics 139 (3): 034307. doi:10.1063/1.4813284. PMID 23883027. Bibcode: 2013JChPh.139c4307Z.
- ↑ Krause, J.; Siriwardane, Upali; Salupo, Terese A.; Wermer, Joseph R. et al. (1993). "Preparation of [Os3(CO)11]2− and its reactions with Os3(CO)12; structures of [Et4N] [HOs3(CO)11] and H2OsS4(CO)". Journal of Organometallic Chemistry 454 (1–2): 263–271. doi:10.1016/0022-328X(93)83250-Y.
- ↑ Carter, Willie J.; Kelland, John W.; Okrasinski, Stanley J.; Warner, Keith E. et al. (1982). "Mononuclear hydrido alkyl carbonyl complexes of osmium and their polynuclear derivatives". Inorganic Chemistry 21 (11): 3955–3960. doi:10.1021/ic00141a019.
- ↑ Brian S. McGilligan; John Arnold; Geoffrey Wilkinson; Bilquis Hussain-Bates; Michael B. Hursthouse (1990). "Reactions of Dimesityldioxo-Osmium(VI) with Donor Ligands; Reactions of MO2(2,4,6-Me3C6H2)2, M = Os or Re, with Nitrogen Oxides. X-Ray Crystal Structures of [2,4,6-Me3C6H2N2]+[OsO2(ONO2)2(2,4,6-Me3C6H2)]–, OsO(NBut)(2,4,6-Me3C6H2)2, OsO3(NBut), and ReO3[N(2,4,6-Me3C6H2)2]". J. Chem. Soc., Dalton Trans. (8): 2465–2475. doi:10.1039/DT9900002465.
- ↑ Weeks, M. E. (1968). Discovery of the Elements (7 ed.). Journal of Chemical Education. pp. 414–418. ISBN 978-0-8486-8579-9. OCLC 23991202. https://archive.org/details/discoveryofeleme0000week.
- ↑ Girolami, Gregory (2012). "Osmium weighs". Nature Chemistry 4 (11): 954. doi:10.1038/nchem.1479. PMID 23089872. Bibcode: 2012NatCh...4..954G.
- ↑ Cotton and Wilkinson, Advanced Inorganic Chemistry, p.1002
- ↑ 15.0 15.1 Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. pp. 671–673, 710. ISBN 978-0-13-039913-7.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Kiernan, J. A.. "Re: "Disposal" of Osmium Tetroxide "Waste"". Department of Anatomy & Cell Biology, The University of Western Ontario. http://www.histosearch.com/histonet/Nov00A/Re.quotDisposalquotofOsmi.html.
- ↑ A. F. Holleman; E. Wiberg (2001). Inorganic chemistry. Academic Press. p. 1465. ISBN 0-12-352651-5.
- ↑ Thiele G.; Woditsch P. (1969). "Neutronenbeugungsuntersuchungen am Osmium(IV)-oxid". Journal of the Less Common Metals 17 (4): 459. doi:10.1016/0022-5088(69)90074-5.
- ↑ J. E. Greedan; D. B. Willson; T. E. Haas (1968). "Metallic nature of osmium dioxide". Inorg. Chem. 7 (11): 2461–2463. doi:10.1021/ic50069a059.
- ↑ Yen, P (2004). "Growth and characterization of OsO2 single crystals". Journal of Crystal Growth 262 (1–4): 271. doi:10.1016/j.jcrysgro.2003.10.021.
- ↑ Boman C.E.; Danielsen, Jacob; Haaland, Arne; Jerslev, Bodil; Schäffer, Claus Erik; Sunde, Erling; Sørensen, Nils Andreas (1970). "Precision Determination of the Crystal Structure of Osmium Dioxide". Acta Chemica Scandinavica 24: 123–128. doi:10.3891/acta.chem.scand.24-0123.
- ↑ Smith, I.C., B.L. Carson, and T.L. Ferguson (1974). "Osmium: An appraisal of environmental exposure.". Env Health Perspect (National Institute of Environmental Health Sciences) 8: 201–213. doi:10.2307/3428200. PMID 4470919.
- ↑ CRC Handbook of Chemistry and Physics, 90th Edition, CRC Press, Boca Raton, Florida, 2009, ISBN:978-1-4200-9084-0, Section 4, Physical Constants of Inorganic Compounds, p. 4-85.
- ↑ 25.0 25.1 Drews, T.; Supeł, J.; Hagenbach, A.; Seppelt, K. (2006). "Solid State Molecular Structures of Transition Metal Hexafluorides". Inorganic Chemistry 45 (9): 3782–3788. doi:10.1021/ic052029f. PMID 16634614.
- ↑ Paine, R. T. (1 June 1973). "Partial hydrolysis of rhenium and osmium hexafluorides. An improved synthesis and characterization of rhenium oxide tetrafluoride". Inorganic Chemistry 12 (6): 1457–1458. doi:10.1021/ic50124a060.
- ↑ Holloway, John H.; Mitchell, S. J. (1971). "Preparation and Crystal Structure of Osmium Pentafluoride". Journal of the Chemical Society: 2789–94. doi:10.1039/J19710002789.
- ↑ Otto Ruff and Ferd. Bornemann (1910). "Über das Osmium, seine analytische Bestimmung, seine Oxyde und seine Chloride". Zeitschrift für anorganische Chemie 65: 429–456. doi:10.1002/zaac.19100650126. https://zenodo.org/record/1428118.
- ↑ Cotton, S. A. (1997). Chemistry of Precious Metals. London: Chapman and Hall. ISBN 0-7514-0413-6.
- ↑ Wells A.F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford Science Publications. ISBN 0-19-855370-6.
- ↑ Paul Machmer (1967). "On the polymorphism of osmium tetrachloride". Chem. Commun. (12): 610a. doi:10.1039/C1967000610A.
- ↑ Thiele, G.; Wochner, H.; Wagner, H. (1985). "Über Osmiumbromide". Zeitschrift für Anorganische und Allgemeine Chemie 530 (11): 178–186. doi:10.1002/zaac.19855301121.
- ↑ Köhler, J. (2014). "Halides: Solid-State Chemistry". Encyclopedia of Inorganic and Bioinorganic Chemistry. pp. 1–22. doi:10.1002/9781119951438.eibc0078.pub2. ISBN 978-1-119-95143-8.
- ↑ 34.0 34.1 34.2 34.3 Fergusson, J. E.; Robinson, B. H.; Roper, W. R. (1962). "405. Iodides of osmium and rhenium". Journal of the Chemical Society (Resumed): 2113. doi:10.1039/JR9620002113.
- ↑ 35.0 35.1 35.2 George K. Schweitzer; Lester L. Pesterfield (2009) (in en) (Ebook). The Aqueous Chemistry of the Elements. Oxford University Press. p. 321. ISBN 978-0-19-974219-6. https://books.google.com/books?id=-TNhhlGcCzwC. Retrieved 11 November 2021.
- ↑ "The Chemistry of Osmium". the Sciences (Scientific American) 35 (904supp): pp. 14453–14454. 1893. doi:10.1038/scientificamerican04291893-14453supp.
- ↑ 37.0 37.1 37.2 Cumberland, Robert W. (April 27, 2005). "Osmium Diboride, An Ultra-Incompressible, Hard Material". Journal of the American Chemical Society 127 (20): 7264–5. doi:10.1021/ja043806y. PMID 15898746.
- ↑ M. Hebbache (2006). "A new superhard material: Osmium diboride OsB2". Solid State Communications 139 (5): 227–231. doi:10.1016/j.ssc.2006.05.041. Bibcode: 2006SSCom.139..227H.
- ↑ Kempter, C. P.; Fries, R. J. (1961). "Crystallography of the Ru-B and Os-B Systems". The Journal of Chemical Physics 34 (6): 1994. doi:10.1063/1.1731807. Bibcode: 1961JChPh..34.1994K.
- ↑ Roof, R. B.; Kempter, C. P. (1962). "New Orthorhombic Phase in the Ru-B and Os-B Systems". The Journal of Chemical Physics 37 (7): 1473. doi:10.1063/1.1733309. Bibcode: 1962JChPh..37.1473R.
- ↑ Xie, Zhilin; Blair, Richard G.; Orlovskaya, Nina; Cullen, David A.; Andrew Payzant, E. (2014-11-01). "Thermal stability of hexagonal OsB2". Journal of Solid State Chemistry 219: 210–219. doi:10.1016/j.jssc.2014.07.035. Bibcode: 2014JSSCh.219..210X.
 |