Chemistry:Sodium pertechnetate
| |
| |
| Names | |
|---|---|
| IUPAC name
Sodium technetate(VII)
| |
| Other names
sodium tetraoxotechnetate (VII)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
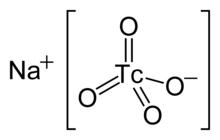
| NaTcO4 | |
| Molar mass | 169.89 g/mol |
| Appearance | White or pale pink solid |
| Melting point | < 1,063 K (790 °C; 1,454 °F)[1] |
| Soluble | |
| Structure[1] | |
| Scheelite | |
| I41/a | |
a = 5.3325(1) Å, c = 11.8503(3) Å
| |
Formula units (Z)
|
4 |
| Related compounds | |
Other anions
|
Sodium permanganate; sodium perrhenate |
Other cations
|
Ammonium pertechnetate |
Related compounds
|
Technetium heptoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium pertechnetate is the inorganic compound with the formula NaTcO4. This colourless salt contains the pertechnetate anion, TcO−4 that has slightly distorted tetrahedron symmetry both at 296 K and at 100 K[2] while the coordination polyhedron of the sodium cation is different from typical for scheelite structure. The radioactive 99mTcO−4 anion is an important radiopharmaceutical for diagnostic use. The advantages to 99mTc include its short half-life of 6 hours and the low radiation exposure to the patient, which allow a patient to be injected with activities of more than 30 millicuries.[3] Na[99mTcO4] is a precursor to a variety of derivatives that are used to image different parts of the body.
Chemistry
TcO−4 is the starting material for most of the chemistry of technetium. Pertechnetate salts are usually colorless.[4] TcO−4 is produced by oxidizing technetium with nitric acid or with hydrogen peroxide. The pertechnetate anion is similar to the permanganate anion but is a weaker oxidizing agent. It is tetrahedral and diamagnetic. The standard electrode potential for TcO−4/TcO2 is only +0.738 V in acidic solution, as compared to +1.695 V for MnO−4/MnO2.[3] Because of its diminished oxidizing power, TcO−4 is stable in alkaline solution. TcO−4 is more similar to ReO−4. Depending on the reducing agent, TcO−4 can be converted to derivatives containing Tc(VI), Tc(V), and Tc(IV).[5] In the absence of strong complexing ligands, TcO−4 is reduced to a +4 oxidation state via the formation of TcO2 hydrate.[3]
Pharmaceutical use
The half-life of 99mTc is long enough that labelling synthesis of the radiopharmaceutical and scintigraphic measurements can be performed without significant loss of radioactivity.[3] The energy emitted from 99mTc is 140 keV, which allows for the study of deep body organs. Radiopharmaceuticals have no intended pharmacologic effect and are used in very low concentrations. Radiopharmaceuticals containing 99mTc are currently being applied in the determining morphology of organs, testing of organ function, and scintigraphic and emission tomographic imaging. The gamma radiation emitted by the radionuclide allows organs to be imaged in vivo tomographically. Currently, over 80% of radiopharmaceuticals used clinically are labelled with 99mTc. A majority of radiopharmaceuticals labelled with 99mTc are synthesized by the reduction of the pertechnetate ion in the presence of ligands chosen to confer organ specificity of the drug. The resulting 99mTc compound is then injected into the body and a "gamma camera" is focused on sections or planes in order to image the spatial distribution of the 99mTc.
Specific imaging applications
99mTc is used primarily in the study of the thyroid gland - its morphology, vascularity, and function. TcO−4 and iodide, due to their comparable charge/radius ratio, are similarly incorporated into the thyroid gland. The pertechnetate ion is not incorporated into the thyroglobulin. It is also used in the study of blood perfusion, regional accumulation, and cerebral lesions in the brain, as it accumulates primarily in the choroid plexus.
Sodium pertechnetate cannot pass through the blood–brain barrier. In addition to the salivary and thyroid glands, 99mTcO−4 localizes in the stomach. 99mTcO−4 is renally eliminated for the first three days after being injected. After a scanning is performed, it is recommended that a patient drink large amounts of water in order to expedite elimination of the radionuclide.[6] Other methods of 99mTcO−4 administration include intraperitoneal, intramuscular, subcutaneous, as well as orally. The behavior of the 99mTcO−4 ion is essentially the same, with small differences due to the difference in rate of absorption, regardless of the method of administration.[7]
Other reactions involving the pertechnetate ion
- Radiolysis of TcO−4 in nitrate solutions proceeds through the reduction to TcO2−4 which induces complex disproportionation processes:
- Pertechnetate can be reduced by H2S to give Tc2S7.[8]
- Pertechnetate is also reduced to Tc(IV/V) compounds in alkaline solutions in nuclear waste tanks without adding catalytic metals, reducing agents, or external radiation. Reactions of mono- and disaccharides with 99mTcO−4 yield Tc(IV) compounds that are water-soluble.[9]
References
- ↑ 1.0 1.1 German, Konstantin E.; Grigoriev, Mikhail S.; Garashchenko, Bogdan L.; Kopytin, Alexander V.; Tyupina, Ekaterina A. (1 July 2017). "Redetermination of the crystal structure of NaTcO4 at 100 and 296 K based on single-crystal X-ray data". Acta Crystallographica Section E 73 (7): 1037–1040. doi:10.1107/S2056989017008362. PMID 28775877.
- ↑ German, K. E.; Grigoriev, M. S.; Garashchenko, B. L.; Kopytin, A. V.; Tyupina, E. A. (2017-07-01). "Redetermination of the crystal structure of NaTcO4 at 100 and 296 K based on single-crystal X-ray data" (in en). Acta Crystallographica Section E: Crystallographic Communications 73 (7): 1037–1040. doi:10.1107/S2056989017008362. ISSN 2056-9890. PMID 28775877. PMC 5499285. https://scripts.iucr.org/cgi-bin/paper?wm5391.
- ↑ 3.0 3.1 3.2 3.3 Schwochau, K. (1994). "Technetium Radiopharmaceuticals-Fundamentals, Synthesis, Structure, and Development". Angew. Chem. Int. Ed. Engl. 33 (22): 2258–2267. doi:10.1002/anie.199422581.
- ↑ Wells, A. F.; Structural Inorganic Chemistry; Clarendon Press: Oxford, Great Britain; 1984; p. 1050.
- ↑ Encyclopædia Britannica: Technetium
- ↑ Shukla, S. K., Manni, G. B., and Cipriani, C. (1977). "The Behaviour of the Pertechnetate Ion in Humans". Journal of Chromatography B 143 (5): 522–526. doi:10.1016/S0378-4347(00)81799-5. PMID 893641.
- ↑ Razzak, M. A.; Naguib, M.; El-Garhy, M. (1967). "Fate of Sodium Pertechnetate-Technetium-99m". Journal of Nuclear Medicine 8 (1): 50–59. PMID 6019138.
- ↑ Emeléus, H. J.; Sharpe, A. G. (1968). Advances in Inorganic Chemistry and Radiochemistry, Volume 11. Academic Press. pp. 26. ISBN 978-0-08-057860-6. https://books.google.com/books?id=-SnCsg5jM_kC&pg=PA26.
- ↑ D. E. Berning, N. C. Schroeder and R. M. Chamberlin (2005). "The autoreduction of pertechnetate in aqueous, alkaline solutions". Journal of Radioanalytical and Nuclear Chemistry 263 (3): 613–618. doi:10.1007/s10967-005-0632-x. https://zenodo.org/record/1232814.
 |
