Chemistry:Platinum(IV) bromide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Platinum(IV) bromide
| |
| Other names
Platinic bromide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| PtBr4 | |
| Molar mass | 514.694 g/mol |
| Appearance | brownish-black crystals |
| Melting point | decomposes at 180°C |
| 0.41 g/100mL @ 20°C | |
| Solubility | slightly soluble in ethanol, diethyl ether[1] |
| Hazards | |
| Safety data sheet | [1] |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | non-flammable |
| Related compounds | |
Other anions
|
Platinum(IV) fluoride Platinum(IV) chloride Platinum(IV) iodide |
Other cations
|
Nickel(II) bromide Palladium(II) bromide |
Related compounds
|
Platinum(II) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Platinum(IV) bromide is the inorganic compound with the formula PtBr4. It is a brown solid. It is a little-used compound mainly of interest for academic research.[2] It is a component of a reagent used in qualitative inorganic analysis.[3]
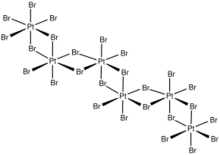
In terms of structure, the compound is an inorganic polymer consisting of interconnected PtBr6 octahedra.
References
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), CRC Press, pp. 481, ISBN 0-8493-0594-2, https://books.google.com/books?id=lFjg0L-uOxoC&q=%22Platinum(IV)+bromide%22&pg=PT866, retrieved 2008-06-19
- ↑ Lagrow, Alec P.; Knudsen, Kristian Rahbek; Alyami, Noktan M.; Anjum, Dalaver H.; Bakr, Osman M. (2015). "Effect of Precursor Ligands and Oxidation State in the Synthesis of Bimetallic Nano-Alloys". Chemistry of Materials 27 (11): 4134–4141. doi:10.1021/acs.chemmater.5b01247.
- ↑ Wenger, P. E. (2007), Reagents for Qualitative Inorganic Analysis, Read Country Books, pp. 242, ISBN 978-1-4067-4847-5, https://books.google.com/books?id=SRAR3FOYxhEC&q=%22Platinum(IV)+bromide%22&pg=PA360, retrieved 2008-06-18
 |


