Chemistry:Krogmann's salt
| Names | |
|---|---|
| IUPAC name
Dipotassium tetracyanoplatinate bromide trihydrate
| |
| Other names
Potassium tetracyanoplatinate bromide trihydrate
| |
| Identifiers | |
| Properties | |
| K2Pt(CN)4Br0.3 | |
| Molar mass | 401.3227 g/mol |
| Appearance | Copper-colored crystalline solid |
| Structure[1] | |
| Tetragonal | |
| 99 (P4mm) | |
a = 9.91 Å, c = 5.78 Å
| |
| Square planar | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Krogmann's salt is a linear chain compound consisting of stacks of tetracyanoplatinate. Sometimes described as molecular wires, Krogmann's salt exhibits highly anisotropic electrical conductivity. For this reason, Krogmann's salt and related materials are of some interest in nanotechnology.[2]
History and nomenclature
Krogmann's salt was first synthesized by Klaus Krogmann in the late 1960s.[3]
Krogmann's salt most commonly refers to a platinum metal complex of the formula K2[Pt(CN)4X0.3] where X is usually bromine (or sometimes chlorine). Many other non-stoichiometric metal salts containing the anionic complex [Pt(CN)4]n− can also be characterized.
Structure and physical properties
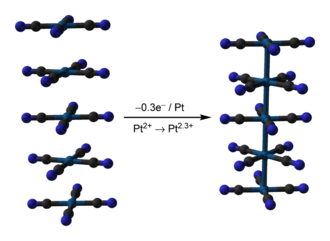
Krogmann's salt is a series of partially oxidized tetracyanoplatinate complexes linked by the platinum-platinum bonds on the top and bottom faces of the planar [Pt(CN)4]n− anions. This salt forms infinite stacks in the solid state based on the overlap of the dz2 orbitals.[2]
Krogmann's salt has a tetragonal crystal structure with a Pt-Pt distance of 2.880 angstroms, which is much shorter than the metal-metal bond distances in other planar platinum complexes such as Ca[Pt(CN)4]·5H2O (3.36 angstroms), Sr[Pt(CN)4]·5H2O (3.58 angstroms), and Mg[Pt(CN)4]·7H2O (3.16 angstroms).[3][4][1] The Pt-Pt distance in Krogmann's salt is only 0.1 angstroms longer than in platinum metal.[5]
Each unit cell contains a site for Cl−, corresponding to 0.5 Cl− per Pt.[1] However, this site is only filled 64% of the time, giving 0.32 Cl− per Pt in the actual compound. Because of this, the oxidation number of Pt does not rise above +2.32.[3]
Krogmann's salt has no recognizable phase range and is characterized by broad and intense intervalence bands in its electronic spectra.[6]
Chemical properties
One of the most widely researched properties of Krogmann's salt is its unusual electric conductance. Because of its linear chain structure and overlap of the platinum orbitals, Krogmann's salt is an excellent conductor of electricity.[2] This property makes it an attractive material for nanotechnology.[7]
Preparation
The usual preparation of Krogmann's salt involves the evaporation of a 5:1 molar ratio mixture of the salts K2[Pt(CN)4] and K2[Pt(CN)4Br2] in water to give copper-colored needles of K2[Pt(CN)4]Br0.32·2.6 H2O.
- 5K2[Pt(CN)4] + K2[Pt(CN)4Br2] + 15.6 H2O → 6K2[Pt(CN)4]Br0.32·2.6 H2O
Because excess PtII or PtIV complex crystallizes out with the product when the reactant ratio is changed, the product is therefore well defined, although non-stoichiometric.[3]
Uses
Krogmann's salt nor any related material has found any commercial applications.
References
- ↑ 1.0 1.1 1.2 Kawasaki, Tatsuji; Jiang, Lei; Iyoda, Tomokazu; Araki, Toshinari; Hashimoto, Kazuhito; Fujishima, Akira (1997). "AFM Molecular Images during Tip-Induced Surface Modification on the (010) Surface of a KCP(Br) Single Crystal" (in en). The Journal of Physical Chemistry B 101 (14): 2723–2729. doi:10.1021/jp962701h. ISSN 1520-6106. https://pubs.acs.org/doi/10.1021/jp962701h.
- ↑ 2.0 2.1 2.2 Bera, J. K.; Dunbar, K. R. (2002). "Chain Compounds Based on Transition Metal Backbones: New Life for an Old Topic". Angew. Chem. Int. Ed. 41 (23): 4453–4457. doi:10.1002/1521-3773(20021202)41:23<4453::AID-ANIE4453>3.0.CO;2-1. PMID 12458505.
- ↑ 3.0 3.1 3.2 3.3 Krogmann, K. (1969). "Planare Komplexe mit Metall-Metall-Bindungen" (in de). Angew. Chem. 81 (1): 10–17. doi:10.1002/ange.19690810103. Bibcode: 1969AngCh..81...10K. Krogmann, K. (1969). "Planar Complexes Containing Metal-Metal Bonds". Angew. Chem. Int. Ed. Engl. 8 (1): 35–42. doi:10.1002/anie.196900351.
- ↑ Krogmann, K.; Hausen, H. D. Z. (1968). "Pt-Chain Structures. 1. Potassium Tetracyanoplatinate Violets K2[Pt(CN)4]X0,3·2,5H2O (X=Cl,Br)". Z. Anorg. Allg. Chem. 358: 67. doi:10.1002/zaac.19683580108.
- ↑ Heger, G.; Deiseroth, H.J.; Schulz, H. "Combined X-ray and neutron diffraction study of K2 (Pt(CN)4)X0.3.3(H2O) with X= Br, Cl (KCP) between 31 K and room temperature" Acta Crystallographica B 1982, volume 24,1968-38. (1978) 34, p725-p731.
- ↑ Clar, R. J. H.; Cround, V. B.; Khokhar, A. R. (1987). "Neutral chain chloride- and bromide-bridged platinum(II,IV) complexes of 1,2-diaminocyclohexane: synthesis and electronic, infrared, Raman, and resonance Raman studies". Inorg. Chem. 26 (20): 3284–3290. doi:10.1021/ic00267a014.
- ↑ Wu, D. Y.; Zhang, T. L. (2004). "Recent developments in linear chain clusters of low-valent platinum group metals" (in zh). Prog. Chem. 16 (6): 911–917.
 |

