Chemistry:Sodium hexachloroplatinate
| |

| |
| Names | |
|---|---|
| Other names
Sodium chloroplatinate
Disodium platinum hexachloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
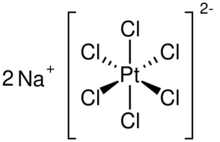
| Na2PtCl6 | |
| Molar mass | 453.7742 g/mol (anhydrous) 561.86588 g/mol (hexahydrate) |
| Appearance | Orange crystalline solid |
| Density | 2.5 g/cm3 |
| Melting point | 110 °C (230 °F; 383 K) |
| Soluble | |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Warning |
| H300, H301, H317, H318, H334 | |
| P261, P264, P270, P272, P280, P285, P301+310, P302+352, P304+341, P305+351+338, P310, P321, P330, P333+313, P342+311, P363, P405, P501 | |
| Related compounds | |
Other anions
|
Sodium hexafluorophosphate Sodium hexafluoroaluminate |
Other cations
|
Potassium hexachloroplatinate Ammonium hexachloroplatinate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium hexachloroplatinate(IV), the sodium salt of chloroplatinic acid, is an inorganic compound with the formula Na2[PtCl6], consisting of the sodium cation and the hexachloroplatinate anion. As explained by Cox and Peters, anhydrous sodium hexachloroplatinate, which is yellow, tends to form the orange hexahydrate upon storage in humid air. The latter can be dehydrated upon heating at 110 °C.[1]
The compound is utilised as the most common chemical shift reference in platinum-195 NMR spectroscopy, relative to which the shifts of other platinum species in solution are reported.[2]
Preparation and reactions
Sodium hexachloroplatinate is obtained as an intermediate in the preparation of Pt complexes, often starting with the dissolution of platinum in aqua regia, giving hexachloroplatinic acid, which is then reacted with sodium chloride and evaporated, leaving the salt behind.[3]
- Pt + 4 HNO3 + 6 HCl → H2[PtCl6] + 4 NO2 + 4 H2O
- H2[PtCl6] + 2 NaCl → Na2[PtCl6] + 2 HCl
The compound can be converted back to platinum metal via conversion to the ammonium salt followed by thermal decomposition, allowing platinum metal to be recovered from laboratory residues.
- Na2[PtCl6] + 2 NH4Cl → (NH4)2[PtCl6] + 2 NaCl
- 3 (NH4)2[PtCl6] → 3 Pt + 2 N2 + 2 NH4Cl + 16 HCl
This compound also reacts with a base, such as sodium hydroxide, producing [Pt(OH)6]−2 ion.[4]
Applications
A 1.2 M solution of sodium hexachloroplatinate in D2O is the most commonly chosen reference compound for chemical shifts in 195Pt NMR. The salt is chosen as it is commercially available at a lower price relative to other platinum compounds, and it possesses high solubility enabling quick acquisition of spectra.[2]
References
- ↑ Cox, Lawrence E.; Peters, Dennis G. (1972). "Disodium Hexachloroplatinate(IV)". Inorganic Syntheses. 13. pp. 173–176. doi:10.1002/9780470132449.ch34. ISBN 9780470132449.
- ↑ 2.0 2.1 Priqueler, Julien R. L.; Butler, Ian S.; Rochon, Fernande D. (2006). "An Overview of 195 Pt Nuclear Magnetic Resonance Spectroscopy" (in en). Applied Spectroscopy Reviews 41 (3): 185–226. doi:10.1080/05704920600620311. ISSN 0570-4928. Bibcode: 2006ApSRv..41..185P. http://www.tandfonline.com/doi/abs/10.1080/05704920600620311.
- ↑ Kauffman, George B.; Teter, Larry A. (1963). "Recovery of Platinum from Laboratory Residues". Inorganic Syntheses. 7. pp. 232–236. doi:10.1002/9780470132388.ch61. ISBN 9780470132388.
- ↑ Vasilchenko, Danila; Berdyugin, Semen; Komarov, Vladislav; Sheven, Dmitriy; Kolesov, Boris; Filatov, Evgeny; Tkachev, Sergey (2022). "Hydrolysis of [PtCl6]2− in Concentrated NaOH Solutions". Inorg. Chem. 61 (15): 5926–5942. doi:10.1021/acs.inorgchem.2c00414. PMID 35380806.
 |

