Chemistry:Platinum disulfide
From HandWiki
(Redirected from Chemistry:Platinum(IV) sulfide)
| |
| Names | |
|---|---|
| IUPAC name
Platinum(IV) sulfide
| |
| Other names
Platinic sulfide
Platinum disulfide dithioxoplatinum | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| PtS2 | |
| Molar mass | 252.9 g/mol |
| Appearance | black solid |
| Density | 7.86 g/cm3 |
| Related compounds | |
Related compounds
|
Platinum(II) sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
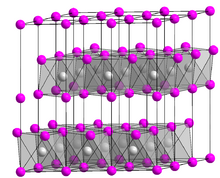
Platinum disulfide is the inorganic compound with the formula PtS2. It is a black, semiconducting solid, which is insoluble in all solvents. The compound adopts the cadmium iodide structure, being composed of sheets of octahedral Pt and pyramidal sulfide centers. Single crystals are grown by chemical vapor transport using phosphorus as the transport agent.[1] A related compound is platinum(II) sulfide, PtS.
References
- ↑ S. Soled; A. Wold (1979). "Platinum Disulfide and Platinum Ditelluride". Inorganic Syntheses 19: 49–51. doi:10.1002/9780470132500.ch9. ISBN 978-0-470-13250-0.
 |

