Chemistry:Resmetirom
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Other names | MGL-3196 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
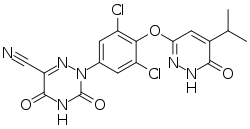
| Formula | C17H12Cl2N6O4 |
| Molar mass | 435.22 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Resmetirom is an experimental drug for the treatment of non-alcoholic steatohepatitis (NASH).[1][2] It is a selective agonist of thyroid hormone receptor-β which increases hepatic fat metabolism and reduces lipotoxicity.[1]
As of 2023, it is in Phase III clinical trials.[3][4]
References
- ↑ 1.0 1.1 "Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial". Lancet 394 (10213): 2012–2024. November 2019. doi:10.1016/S0140-6736(19)32517-6. PMID 31727409.
- ↑ "Non-Alcoholic Fatty Liver Disease (NAFLD) Marketed and Pipeline Drugs Assessment, Clinical Trials and Competitive Landscape". Yahoo! Finance. January 23, 2023. https://finance.yahoo.com/news/non-alcoholic-fatty-liver-disease-010500736.html.
- ↑ "A 52-Week Phase 3 Clinical Trial of Resmetirom in 180 Patients with Well-Compensated NASH Cirrhosis". AASLD: The Liver Meeting. https://www.aasld.org/the-liver-meeting/52-week-phase-3-clinical-trial-resmetirom-180-patients-well-compensated-nash.
- ↑ "Madrigal Announces Positive Topline Results from the Pivotal Phase 3 MAESTRO-NASH Clinical Trial of Resmetirom for the Treatment of NASH and Liver Fibrosis" (Press release). Madrigal Pharmaceuticals. December 19, 2022.
 |

