Chemistry:Selenocystine
From HandWiki
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| 1969559 | |
| ChEBI |
|
| ChemSpider | |
| EC Number |
|
| KEGG |
|
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C6H12N2O4Se2 | |
| Molar mass | 334.114 g·mol−1 |
| Appearance | white solid |
| Melting point | 222 °C (432 °F; 495 K) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H301, H331, H373, H410 | |
| P260, P261, P264, P270, P271, P273, P301+316Script error: No such module "Preview warning".Category:GHS errors, P304+340, P316Script error: No such module "Preview warning".Category:GHS errors, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P330, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
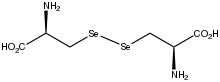
Selenocystine is the amino acid with the formula (HO
2CCH(NH
2)CH
2Se)
2. It is the oxidized derivative of the canonical amino acid selenocysteine (HO
2CCH(NH
2)CH
2SeH). The compound can also be prepared synthetically from serine.[2] Because selenocysteine is not easily isolated or handled, it is often generated by reduction of selenocystine in situ.[3] The selenium–selenium bond length is 2.321 Å, which is 14% longer than the disulfide bond in cystine at 2.040 Å.[4]
References
- ↑ "L-Selenocystine" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/207306#section=Safety-and-Hazards.
- ↑ Muttenthaler, Markus; Alewood, Paul F. (2008). "Selenopeptide chemistry". Journal of Peptide Science 14 (12): 1223–1239. doi:10.1002/psc.1075. PMID 18951416.
- ↑ Tapiero, H.; Townsend, D.M; Tew, K.D (2003). "The antioxidant role of selenium and seleno-compounds". Biomedicine & Pharmacotherapy 57 (3–4): 134–144. doi:10.1016/S0753-3322(03)00035-0. PMID 12818475.
- ↑ Görbitz, Carl Henrik; Levchenko, Vladimir; Semjonovs, Jevgenijs; Sharif, Mohamed Yusuf (2015). "Crystal structure of seleno-L-cystine dihydrochloride". Acta Crystallogr. E 71 (Pt 6): 726–729. doi:10.1107/S205698901501021X. PMID 26090162.
 |