Chemistry:Ulotaront
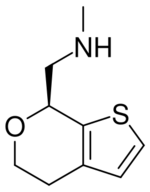 | |
| Clinical data | |
|---|---|
| Other names | SEP-363856; SEP-856 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C9H13NOS |
| Molar mass | 183.27 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ulotaront (INN;[1] developmental codes SEP-363856, SEP-856) is an investigational antipsychotic that is undergoing clinical trials for the treatment of schizophrenia and Parkinson's disease psychosis.[2][3] The medication was discovered in collaboration between PsychoGenics Inc. and Sunovion Pharmaceuticals[2] (which was subsequently merged into Sumitomo Pharma[4]) using PsychoGenics' behavior and AI-based phenotypic drug discovery platform, SmartCube.[5] Ulotaront is in Phase III of clinical development.
Research has shown that ulotaront results in a greater reduction from baseline in the PANSS total score than placebo.[6] Treatment with ulotaront, as compared with placebo, was also associated with an improvement in sleep quality.[6] Ulotaront was awarded a Breakthrough Therapy designation due to its increased efficacy and greatly reduced side effects compared to current treatments.[7]
Adverse effects
The adverse effect profile of ulotaront differs from that of other antipsychotics because its mechanism of action does not involve antagonism of dopamine receptors in the brain, which is responsible for the drug-induced movement disorders (like akathisia) that may occur with those agents.[8] Some adverse events reported in preliminary clinical trials are somnolence, agitation, nausea, diarrhea, and dyspepsia.[8]
Pharmacology
Mechanism of action
The mechanism of action of ulotaront in the treatment of schizophrenia is unclear. However, it is thought to be an agonist at the trace amine-associated receptor 1 (TAAR1) and serotonin 5-HT1A receptors.[2][9] This mechanism of action is unique among available antipsychotics, which generally antagonize dopamine receptors (especially dopamine D2 receptor).[10][11]
Pharmacokinetics
The precise pharmacokinetic profile of ulotaront has not been reported, though the developer has suggested that the pharmacokinetic data supports once daily dosing.[9]
Research
As of 2018, Sunovion, the maker of another antipsychotic called lurasidone (Latuda), is conducting clinical trials on ulotaront in partnership with the preclinical research company PsychoGenics.[3][12][13] The U.S. Food and Drug Administration has granted ulotaront the breakthrough therapy designation.[9][14] In addition to schizophrenia, ulotaront is also being studied for the treatment of psychosis associated with Parkinson's disease.[14]
The Brief Negative Symptom Scale (BNSS) has been used to assess the effect of Ulotaront on the negative symptoms of schizophrenia.[15]
In July 2023, the pharmaceutical company behind the drug announced that the drug had failed to outperform placebo in the treatment of acutely psychotic patients with schizophrenia, as measured by the PANSS.[16]
See also
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN)". WHO Drug Information 34 (3). 2020. https://cdn.who.int/media/docs/default-source/international-nonproprietary-names-(inn)/pl124.pdf?sfvrsn=6437f035_10&download=true. "Proposed INN: List 124 – COVID-19 (special edition)".
- ↑ 2.0 2.1 2.2 "SEP 363856". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800036955.
- ↑ 3.0 3.1 "New Psychotropic Drug for Schizophrenia Promising in Early Testing". Reuters Health Information. https://www.medscape.com/viewarticle/906892?src=wnl_edit_tpal&uac=194606CT&impID=1846550&faf=1.
- ↑ "US Sumitomo Pharma Subsidiaries Combine to Form Sumitomo Pharma America". American Pharmaceutical Review. 7 April 2023. https://www.americanpharmaceuticalreview.com/1315-News/595974-US-Sumitomo-Pharma-Subsidiaries-Combine-to-Form-Sumitomo-Pharma-America. Retrieved 10 July 2023.
- ↑ "Sunovion Presents Data From Marketed and Late-Stage Development Psychiatric Compounds At The American Psychiatric Association (APA) Annual Meeting 2021" (in en). 2021-05-03. https://www.businesswire.com/news/home/20210503005385/en/Sunovion-Presents-Data-From-Marketed-and-Late-Stage-Development-Psychiatric-Compounds-At-The-American-Psychiatric-Association-APA-Annual-Meeting-2021.
- ↑ 6.0 6.1 "A Non-D2-Receptor-Binding Drug for the Treatment of Schizophrenia". The New England Journal of Medicine 382 (16): 1497–1506. April 2020. doi:10.1056/NEJMoa1911772. PMID 32294346.
- ↑ "Sunovion and PsychoGenics Announce that SEP-363856 Has Received FDA Breakthrough Therapy Designation for the Treatment of People with Schizophrenia" (in en). 2019-05-10. https://www.businesswire.com/news/home/20190510005212/en/Sunovion-and-PsychoGenics-Announce-that-SEP-363856-Has-Received-FDA-Breakthrough-Therapy-Designation-for-the-Treatment-of-People-with-Schizophrenia.
- ↑ 8.0 8.1 "'Game Changer' for Schizophrenia on the Horizon?". WebMD LLC. https://www.medscape.com/viewarticle/913348.
- ↑ 9.0 9.1 9.2 "Sunovion and PsychoGenics Announce that SEP-363856 Has Received FDA Breakthrough Therapy Designation for the Treatment of People with Schizophrenia". Bloomberg.com (Bloomberg L.P.). 10 May 2019. https://www.bloomberg.com/press-releases/2019-05-10/sunovion-and-psychogenics-announce-that-sep-363856-has-received-fda-breakthrough-therapy-designation-for-the-treatment-of-people.
- ↑ "O12.5. Efficacy and Safety of Sep-363856, A Novel Psychotropic Agent with a Non-D2 Mechanism of Action, in the Treatment of Schizophrenia: A 4-Week, Randomized, Placebo-Controlled Trial". Schizophrenia Bulletin 45 (Suppl 2): S199. 2019. doi:10.1093/schbul/sbz021.269.
- ↑ "SEP-363856, a Novel Psychotropic Agent with a Unique, Non-D2 Receptor Mechanism of Action". The Journal of Pharmacology and Experimental Therapeutics 371 (1): 1–14. October 2019. doi:10.1124/jpet.119.260281. PMID 31371483.
- ↑ "Sunovion – Our Therapies". Sumitomo Dainippon Pharma Co., Ltd.. http://www.sunovion.us/our-therapies/.
- ↑ "About Us". PsychoGenics. http://www.psychogenics.com/index.html.
- ↑ 14.0 14.1 "Drug Receives FDA's Breakthrough Therapy Designation for Treating Individuals with Schizophrenia". Pharmacy & Healthcare Communications, LLC. https://www.pharmacytimes.com/resource-centers/mental-health/drug-receives-fdas-breakthrough-therapy-designation-for-treating-individuals-with-schizophrenia.
- ↑ "The brief negative symptom scale in translation: A review of psychometric properties and beyond". European Neuropsychopharmacology 33: 36–44. April 2020. doi:10.1016/j.euroneuro.2020.01.018. PMID 32081498.
- ↑ "Disappointing Results for Ulotaront in Two Phase 3 Schizophrenia Trials" (in en-US). 2023-08-01. https://www.empr.com/home/news/drugs-in-the-pipeline/disappointing-results-for-ulotaront-in-two-phase-3-schizophrenia-trials/.
 |

