Chemistry:Vaniprevir
From HandWiki
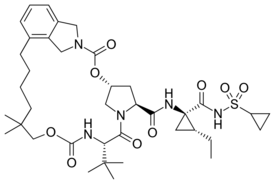
| |
| Names | |
|---|---|
| IUPAC name
(1R,21S,24S)-21-tert-Butyl-N-((1R,2R)-1-{[(cyclopropylsulfonyl)amino]carbonyl}-2-ethylcyclopropyl)-16,16-dimethyl-3,19,22-trioxo-2,18-dioxa-4,20,23-triazatetracyclo[21.2.1.14,7.06,11]-heptacosa-6,8,10-triene-24-carboxamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C38H55N5O9S | |
| Molar mass | 757.94 g·mol−1 |
| Appearance | White powder |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H373 | |
| P260, P314, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Vaniprevir (MK-7009) is a macrocyclic hepatitis C virus (HCV) NS3/4A protease inhibitor, developed by Merck & Co., which is currently in clinical testing.[1] In Japan, it was approved for treating hepatitis C in 2014 under the brand name Vanihep.[2][3]
References
- ↑ "Discovery of vaniprevir (MK-7009), a macrocyclic hepatitis C virus NS3/4a protease inhibitor". J. Med. Chem. 53 (6): 2443–63. March 2010. doi:10.1021/jm9015526. PMID 20163176.
- ↑ "First recommendation for HCV drug vaniprevir, in Japan". datamonitorhealthcare.com. September 25, 2014. http://www.datamonitorhealthcare.com/first-recommendation-for-hcv-drug-vaniprevir-in-japan/.
- ↑ New Drugs Approved. Pharmaceuticals and Medical Devices Agency. http://www.pmda.go.jp/files/000197902.pdf.
 |
