Chemistry:Galidesivir
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| Chemical and physical data | |
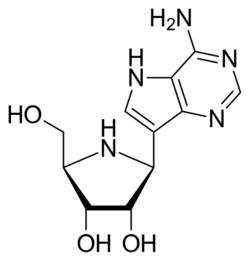
| Formula | C11H15N5O3 |
| Molar mass | 265.273 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Galidesivir (BCX4430, immucillin-A) is an antiviral drug, an adenosine analog[1] (a type of nucleoside analog).[2] It was developed by BioCryst Pharmaceuticals with funding from NIAID, originally intended as a treatment for hepatitis C, but subsequently developed as a potential treatment for deadly filovirus infections such as Ebola virus disease and Marburg virus disease, as well as Zika virus.[3] Currently, galidesivir is under phase 1 human trial in Brazil for coronavirus.[4]
It also shows broad-spectrum antiviral effectiveness against a range of other RNA virus families, including bunyaviruses, arenaviruses, paramyxoviruses, coronaviruses, flaviviruses, and phleboviruses.[5] Galidesivir has been demonstrated to protect against both Ebola and Marburg viruses in both rodents and monkeys, even when administered up to 48 hours after infection,[1] and development for use in humans was then being fast-tracked due to concerns about the lack of treatment options for the 2013-2016 Ebola virus epidemic in West Africa.[6]
Galidesivir later showed efficacy against Zika virus in a mouse model.[7]
Galidesivir abrogates viremia in Zika virus–infected rhesus Macaques.[8]
Galidesivir is one of several antiviral drugs being tested for coronavirus disease 2019.[9]
On April 9, 2020, BioCryst opened enrollment into a randomized, double-blind, placebo-controlled clinical trial to assess the safety, clinical impact and antiviral effects of galidesivir in patients with COVID-19.[4]
See also
- Atoltivimab/maftivimab/odesivimab
- Bemnifosbuvir
- Brincidofovir
- Coronavir
- 3-Deazaneplanocin A
- Favipiravir
- FGI-106
- GS-441524
- JK-05
- Lopinavir/ritonavir
- Lamivudine
- Ansuvimab
- MK-608
- Molnupiravir
- Nelfinavir
- Oseltamivir
- Nirmatrelvir/ritonavir
- Peramivir
- Remdesivir
- Ribavirin
- Ensitrelvir
- TKM-Ebola
- Triazavirin
- Umifenovir
- ZMapp
References
- ↑ 1.0 1.1 "Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430". Nature 508 (7496): 402–405. April 2014. doi:10.1038/nature13027. PMID 24590073. Bibcode: 2014Natur.508..402W.
- ↑ "Potent inhibition of the C-P lyase nucleosidase PhnI by Immucillin-A triphosphate". Biochemistry 52 (42): 7366–7368. October 2013. doi:10.1021/bi4013287. PMID 24111876.
- ↑ BioCryst Pharmaceuticals, Inc. (June 10, 2020). "Galidesivir Stops Zika Viral Replication in Primate Model". GlobeNewswire News Room (Press release).
- ↑ 4.0 4.1 Clinical trial number NCT03891420 for "A Study to Evaluate the Safety, Pharmacokinetics and Antiviral Effects of Galidesivir in Yellow Fever or COVID-19" at ClinicalTrials.gov
- ↑ "Galidesivir limits Rift Valley fever virus infection and disease in Syrian golden hamsters". Antiviral Research 156: 38–45. August 2018. doi:10.1016/j.antiviral.2018.05.013. PMID 29864447.
- ↑ "BioWar Lab Helping To Develop Treatment For Ebola". Forbes Magazine. 8 April 2014. https://www.forbes.com/sites/paulrodgers/2014/08/04/biowar-lab-develops-cure-for-ebola/.
- ↑ "Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model". Antiviral Research 137: 14–22. January 2017. doi:10.1016/j.antiviral.2016.11.003. PMID 27838352.
- ↑ "A direct-acting antiviral drug abrogates viremia in Zika virus-infected rhesus macaques". Science Translational Medicine 12 (547). June 2020. doi:10.1126/scitranslmed.aau9135. PMID 32522808.
- ↑ "Coronavirus outbreak: Vaccines/drugs in the pipeline for Covid-19". clinicaltrialsarena.com. 19 February 2020. https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/.
 |
