Medicine:Alteplase
| Clinical data | |
|---|---|
| Trade names | Activase, Actilyse, Cathflo Activase, others |
| Other names | t-PA, rt-PA |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C2569H3928N746O781S40 |
| Molar mass | 59042.52 g·mol−1 |
| (verify) | |
Alteplase, sold under the brand name Activase among others, is a biosynthetic form of human tissue-type plasminogen activator (t-PA). It is a thrombolytic medication used to treat acute ischemic stroke, acute ST-elevation myocardial infarction (a type of heart attack), pulmonary embolism associated with low blood pressure, and blocked central venous catheter.[5] It is given by injection into a vein or artery.[5] Alteplase is the same as the normal human plasminogen activator produced in vascular endothelial cells[6] and is synthesized via recombinant DNA technology in Chinese hamster ovary cells (CHO). Alteplase causes the breakdown of a clot by inducing fibrinolysis.[7]
It is on the World Health Organization's List of Essential Medicines.[8]
Medical uses
Alteplase is indicated for the treatment of acute ischemic stroke, acute myocardial infarction, acute massive pulmonary embolism, and blocked catheters.[5][2][3] Similar to other thrombolytic drugs, alteplase is used to dissolve clots to restore tissue perfusion, but this can vary depending on the pathology.[9][10] Generally, alteplase is delivered intravenously into the body.[7] To treat blocked catheters, alteplase is administered directly into the catheter.[7]
Ischemic stroke
In adults diagnosed with acute ischemic stroke, thrombolytic treatment with alteplase is the standard of care.[10][11] Administration of alteplase is associated with improved functional outcomes and reduced incidence of disability.[12] Alteplase used in conjunction with mechanical thrombectomy is associated with better outcomes.[13][14]
Pulmonary embolism
As of 2019, alteplase is the most commonly used medication to treat pulmonary embolism (PE).[15] Alteplase has a short infusion time of 2 hours and a half-life of 4–6 minutes.[15] Alteplase has been approved by the FDA, and treatment can be done via systemic thrombolysis or catheter-directed thrombolysis.[15][16]
Systemic thrombolysis can quickly restore right ventricular function, heart rate, and blood pressure in patients with acute PE.[17] However, standard doses of alteplase used in systemic thrombolysis may lead to massive bleeding, such as intracranial hemorrhage, particularly in older patients.[15] A systematic review has shown that low-dose alteplase is safer than and as effective as the standard amount.[18]
Blocked catheters
Alteplase can be used in small doses to clear blood clots that obstruct a catheter, reopening the catheter so it can continue to be used.[3][12] Catheter obstruction is commonly observed with a central venous catheter.[19] Currently, the standard treatment for catheter obstructions in the United States is alteplase administration.[6] Alteplase is effective and low risk for treating blocked catheters in adults and children.[6][19] Overall, adverse effects of alteplase for clearing blood clots are rare.[20] Novel alternatives to treat catheter occlusion, such as tenecteplase, reteplase, and recombinant urokinase, offer the advantage of shorter dwell times than alteplase.[19]
Contraindications
A person should not receive alteplase treatment if testing shows they are not suffering from an acute ischemic stroke or if the risks of treatment outweigh the likely benefits.[10] Alteplase is contraindicated in those with bleeding disorders that increase a person's tendency to bleed and in those with an abnormally low platelet count.[14] Active internal bleeding and high blood pressure are additional contraindications for alteplase.[14] The safety of alteplase in the pediatric population has not been determined definitively.[14] Additional contraindications for alteplase when used specifically for acute ischemic stroke include current intracranial hemorrhage and subarachnoid hemorrhage.[21] Contraindications for use of alteplase in people with a STEMI are similar to those of acute ischemic stroke.[9] People with an acute ischemic stroke may also receive other therapies including mechanical thrombectomy.[10]
Adverse effects
Given that alteplase is a thrombolytic medication, a common adverse effect is bleeding, which can be life-threatening.[22] Adverse effects of alteplase include symptomatic intracranial hemorrhage and fatal intracranial hemorrhage.[22]
Angioedema is another adverse effect of alteplase, which can be life-threatening if the airway becomes obstructed.[2] Other side effects may rarely include allergic reactions.[5]
Mechanism of action
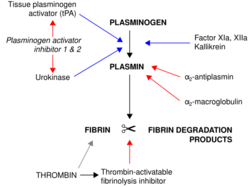
Alteplase binds to fibrin in a blood clot and activates the clot-bound plasminogen.[7] Alteplase cleaves plasminogen at the site of its Arg561-Val562 peptide bond to form plasmin.[7] Plasmin is a fibrinolytic enzyme that cleaves the cross-links between polymerized fibrin molecules, causing the blood clot to break down and dissolve, a process called fibrinolysis.[7]
Regulation and inhibition
Plasminogen activator inhibitor 1 stops alteplase activity by binding to it and forming an inactive complex, which is removed from the bloodstream by the liver.[7] Fibrinolysis by plasmin is extremely short-lived due to plasmin inhibitors, which inactivate and regulate plasmin activity.[7]
History
In 1995, a study by the National Institute of Neurological Disorders and Stroke showed the effectiveness of administering intravenous alteplase to treat ischemic stroke.[23] This sparked a medical paradigm shift as it redesigned stroke treatment in the emergency department to allow for timely assessment and therapy for ischemic stroke patients.[23]
Society and culture
Alteplase was added to the World Health Organization's List of Essential Medicines in 2019, for use in ischemic stroke.[24][25]
Legal status
In May 1987, the United States FDA requested additional data for the drug rather than approve it outright, causing Genentech stock prices to fall by nearly one quarter. The decision was described as a surprise to the company as well as many cardiologists and regulators,[26] and it generated significant criticism of the FDA, including from The Wall Street Journal editorial board.[27][28]
After results from two additional trials were obtained,[27] Alteplase was approved for medical use in the United States in November 1987 for the treatment of myocardial infarction.[5][2][29][30] This was just seven years after the first efforts were made to produce recombinant t-PA, making it one of the fastest drug developments in history.[30]
Economics
The cost of alteplase in the United States increased by 111% between 2005 and 2014, despite there being no proportional increase in the costs of other prescription drugs.[31] However, alteplase continues to be cost-effective.[31]
Brand names
Alteplase is marketed as Actilyse, Activase, and Cathflo or Cathflo Activase.[2][3][32][33]
Controversies
Alteplase is extremely underused in low- and middle-income countries.[34] This may be due to its high cost and the fact that it is often not covered by health insurance.[34]
There may be citation bias in the literature on alteplase in ischemic stroke, as studies reporting positive results for tissue plasminogen activator are more likely to be cited in following studies than those reporting negative or neutral results.[35]
There is a sex difference in the use of intravenous tissue plasminogen activator, as it is less likely to be used for women with acute ischemic stroke than men.[36] However, this difference has been improving since 2008.[36]
References
- ↑ Australian Public Assessment Report for Alteplase (AusPAR) (Report). February 2011. https://www.tga.gov.au/sites/default/files/auspar-actilyse.pdf.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Activase- alteplase kit". 5 December 2018. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c669f77c-fa48-478b-a14b-80b20a0139c2.
- ↑ 3.0 3.1 3.2 3.3 "Cathflo Activase- alteplase injection, powder, lyophilized, for solution". 6 September 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=91ecdef2-95ff-42dd-a31c-c8a09cab3ad9.
- ↑ "Actilyse". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/referrals/actilyse.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Alteplase Monograph for Professionals". https://www.drugs.com/monograph/alteplase.html.
- ↑ 6.0 6.1 6.2 "Management of occlusion and thrombosis associated with long-term indwelling central venous catheters". Lancet 374 (9684): 159–69. July 2009. doi:10.1016/S0140-6736(09)60220-8. PMID 19595350.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 "Tissue Plasminogen Activator". StatPearls. Treasure Island (FL): StatPearls Publishing. April 2020. http://www.ncbi.nlm.nih.gov/books/NBK507917/. Retrieved 10 November 2020.
- ↑ The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. 2023. WHO/MHP/HPS/EML/2023.02.
- ↑ 9.0 9.1 "2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines". Circulation 127 (4): e362-425. January 2013. doi:10.1161/CIR.0b013e3182742cf6. PMID 23247304.
- ↑ 10.0 10.1 10.2 10.3 "Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association". Stroke 50 (12): e344–e418. December 2019. doi:10.1161/STR.0000000000000211. PMID 31662037.
- ↑ Solomon, Caren G., ed (July 2020). "Acute Ischemic Stroke". The New England Journal of Medicine 383 (3): 252–260. doi:10.1056/NEJMcp1917030. PMID 32668115.
- ↑ 12.0 12.1 "Alteplase". StatPearls. Treasure Island (FL): StatPearls Publishing. 2020. http://www.ncbi.nlm.nih.gov/books/NBK499977/. Retrieved 30 October 2020.
- ↑ "Mechanical Thrombectomy Outcomes With and Without Intravenous Thrombolysis in Stroke Patients: A Meta-Analysis". Stroke 48 (9): 2450–2456. September 2017. doi:10.1161/STROKEAHA.117.017320. PMID 28747462.
- ↑ 14.0 14.1 14.2 14.3 "Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association". Stroke 47 (2): 581–641. February 2016. doi:10.1161/STR.0000000000000086. PMID 26696642.
- ↑ 15.0 15.1 15.2 15.3 "Update on Thrombolytic Therapy in Acute Pulmonary Thromboembolism". The Eurasian Journal of Medicine 51 (2): 186–190. June 2019. doi:10.5152/eurasianjmed.2019.19291. PMID 31258361.
- ↑ "Systemic Thrombolysis for Pulmonary Embolism: A Review". P & T 41 (12): 770–775. December 2016. PMID 27990080.
- ↑ "Ultrasound-assisted thrombolysis for acute pulmonary embolism: a systematic review". European Heart Journal 35 (12): 758–64. March 2014. doi:10.1093/eurheartj/ehu029. PMID 24497337.
- ↑ "Lower dosage of recombinant tissue-type plasminogen activator (rt-PA) in the treatment of acute pulmonary embolism: a systematic review and meta-analysis". Thrombosis Research 133 (3): 357–63. March 2014. doi:10.1016/j.thromres.2013.12.026. PMID 24412030.
- ↑ 19.0 19.1 19.2 "Thrombolytic therapy for central venous catheter occlusion". Haematologica 97 (5): 641–50. May 2012. doi:10.3324/haematol.2011.050492. PMID 22180420.
- ↑ "Efficacy, safety, and cost of thrombolytic agents for the management of dysfunctional hemodialysis catheters: a systematic review". Pharmacotherapy 31 (10): 1031–40. October 2011. doi:10.1592/phco.31.10.1031. PMID 21950645.
- ↑ "Changing contraindications for t-PA in acute stroke: review of 20 years since NINDS". Current Cardiology Reports 17 (10): 81. October 2015. doi:10.1007/s11886-015-0633-5. PMID 26277361.
- ↑ 22.0 22.1 "Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials". Lancet 384 (9958): 1929–35. November 2014. doi:10.1016/S0140-6736(14)60584-5. PMID 25106063.
- ↑ 23.0 23.1 "Twenty-Year History of the Evolution of Stroke Thrombolysis With Intravenous Alteplase to Reduce Long-Term Disability". Stroke 46 (8): 2341–6. August 2015. doi:10.1161/STROKEAHA.114.007564. PMID 26152294.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Executive summary: the selection and use of essential medicines 2019: report of the 22nd WHO Expert Committee on the selection and use of essential medicines. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.05. License: CC BY-NC-SA 3.0 IGO.
- ↑ Sun, Marjorie (3 July 1987). "FDA Puts New Heart Drug on Hold: A surprise decision by the FDA to withhold approval of TPA, a potent clot-dissolving drug, highlights a scientific debate among cardiologists". Science 237 (4810): 16–18. doi:10.1126/science.3110948. PMID 3110948.
- ↑ 27.0 27.1 Carpenter, Daniel P. (2010). Reputation and power : organizational image and pharmaceutical regulation at the FDA. Princeton: Princeton University Press. pp. 2–7. ISBN 9780691141794.
- ↑ Sun, Marjorie (28 July 1987). "Heart Drug in Limbo". The Washington Post. https://www.washingtonpost.com/archive/lifestyle/wellness/1987/07/28/heart-drug-in-limbo/64849186-8700-4cbc-bbb1-92580793f30c/.
- ↑ "Activase: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=103172.
- ↑ 30.0 30.1 "The tissue-type plasminogen activator story". Arteriosclerosis, Thrombosis, and Vascular Biology 29 (8): 1151–5. August 2009. doi:10.1161/ATVBAHA.108.179655. PMID 19605778.
- ↑ 31.0 31.1 "Cost of Alteplase Has More Than Doubled Over the Past Decade". Stroke 48 (7): 2000–2002. July 2017. doi:10.1161/strokeaha.116.015822. PMID 28536176.
- ↑ "Cathflo Activase Uses, Side Effects & Warnings". https://www.drugs.com/mtm/cathflo-activase.html.
- ↑ "Tissue-type plasminogen activator: a historical perspective and personal account". Journal of Thrombosis and Haemostasis 2 (4): 541–6. April 2004. doi:10.1111/j.1538-7933.2004.00645.x. PMID 15102005.
- ↑ 34.0 34.1 "Presentation, Evaluation, Management, and Outcomes of Acute Stroke in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis". Neuroepidemiology 51 (1–2): 104–112. 2018. doi:10.1159/000491442. PMID 30025394.
- ↑ "Citation bias favoring positive clinical trials of thrombolytics for acute ischemic stroke: a cross-sectional analysis". Trials 17 (1): 473. September 2016. doi:10.1186/s13063-016-1595-7. PMID 27677444.
- ↑ 36.0 36.1 "Sex differences in IV thrombolysis treatment for acute ischemic stroke: A systematic review and meta-analysis". Neurology 95 (1): e11–e22. July 2020. doi:10.1212/wnl.0000000000009733. PMID 32522796.
 |
