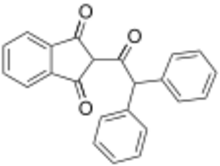
Chemistry:Diphenadione
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-(Diphenylacetyl)-1H-indene-1,3(2H)-dione | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C23H16O3 | |
| Molar mass | 340.378 g·mol−1 |
| Pharmacology | |
| 1=ATC code }} | B01AA10 (WHO) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diphenadione is a vitamin K antagonist that has anticoagulant effects and is used as a rodenticide against rats, mice, voles, ground squirrels and other rodents. The chemical compound is an anti-coagulant with active half-life longer than warfarin and other synthetic 1,3-indandione anticoagulants.[3][4]
It is toxic to mammals, in all forms; exposure and oral ingestion of the toxin may cause irregular heartbeat and major maladies associated with its impact on blood clotting, depending on dose.[5] As a "second-generation" anticoagulant, diphenadione is more toxic than the first generation compounds (e.g., warfarin).[6]:436 For purposes of treating toxicity on exposure, diphenadione is grouped with other vitamin K antagonists (coumarins and indandiones); despite being directed at rodents and being judged as less hazardous to humans and domestic animals than other rodenticides in use[when?] (by the U.S. Environmental Protection Agency), indandione anticoagulants, nevertheless, "may cause human toxicity at a much lower dose than conventional 'first-generation anticoagulants'… and can bioaccumulate in the liver."[7]:173
References
- ↑ Kukovinets, O. S.; Abdullin, M. I.; Zainullin, R. A.; Kunakova, R. V. (2008). Chemical and Physical Methods for Protecting Biopolymers Against Pests. New York: Nova Biomedical Books. p. 185. ISBN 9781604563313. https://books.google.com/books?id=rWfdntu88VUC&q=ratindan&pg=PA185.
- ↑ "Catalog.md". https://www.catalog.md/drugs/ratindan-1.html.
- ↑ 3.0 3.1 EXTOXNET Staff (1993-09-01). "Diphacinone". EXTOXNET. http://pmep.cce.cornell.edu/profiles/extoxnet/dienochlor-glyphosate/diphacinone-ext.html.
- ↑ Meister, R.T. (ed.). 1992. Farm Chemicals Handbook '92. Meister Publishing Company, Willoughby, OH.
- ↑ Bell Laboratories, Inc. July, 1990. Diphacinone Technical: MSDS. Bell Labs, Madison, WI.
- ↑ Murphy, Michael J.; Talcott, Patricia A. (2013). "Anticoagulant Rodenticides (Ch. 32)". in Peterson, Michael E.. Small Animal Toxicology (3rd ed.). St. Louis, MO, US: Elsevier Health Sciences. pp. 435–446, esp. 435–439. ISBN 978-0323241984. https://books.google.com/books?isbn=0323241980. Retrieved 5 April 2016.
- ↑ Reigart, J. Routt & Roberts, James R. (Eds.) (2013). "Rodenticides (Ch. 18, § Coumarins and Indandiones)". Recognition and Management of Pesticide Poisonings (6th ed.). Corvallis, OR, US: National Pesticide Information Center (Oregon State University and the U.S. Environmental Protection Agency. http://npic.orst.edu/RMPP/rmpp_ch18.pdf. Retrieved 5 April 2016. "The first-generation anticoagulants, for example, are reasonably effective against pest rodents and are less toxic than second-generation anticoagulants… / Very small amounts of the extremely toxic rodenticides sodium fluoroacetate, fluoracetamide, strychnine, crimidine, yellow phosphorus, zinc phosphide and thallium sulfate can cause severe and even fatal poisoning. Cholecalciferol is also a highly toxic agent. The anticoagulants, indandiones and red squill, are less hazardous to humans and domestic animals. Some of the newer anticoagulant compounds, termed 'second-generation anticoagulants,' may cause human toxicity at a much lower dose than conventional 'first-generation anticoagulants'… and can bioaccumulate in the liver…" [p. 173, emphasis in source].
Further reading
- Reigart, J. Routt & Roberts, James R. (Eds.) (2013). "Rodenticides (Ch. 18, § Coumarins and Indandiones)". Recognition and Management of Pesticide Poisonings (6th ed.). Corvallis, OR, US: National Pesticide Information Center (Oregon State University and the U.S. Environmental Protection Agency. pp. 173–187. http://npic.orst.edu/RMPP/rmpp_ch18.pdf. Retrieved 5 April 2016. A safety handbook that explains how incidents of poisoning by various rodenticides are treated.
 |

