Medicine:Temporal lobe epilepsy
| Temporal lobe epilepsy | |
|---|---|
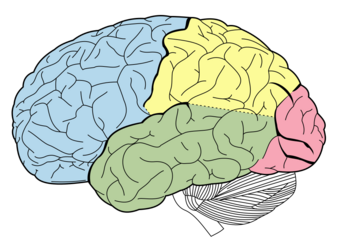 | |
| Lobes of the brain. Temporal lobe in green | |
| Specialty | Neurology, Psychiatry |
In the field of neurology, temporal lobe epilepsy is an enduring brain disorder that causes unprovoked seizures from the temporal lobe. Temporal lobe epilepsy is the most common type of focal onset epilepsy among adults.[1] Seizure symptoms and behavior distinguish seizures arising from the medial temporal lobe from seizures arising from the lateral (neocortical) temporal lobe.[2] Memory and psychiatric comorbidities may occur. Diagnosis relies on electroencephalographic (EEG) and neuroimaging studies.[3][4] Anticonvulsant medications, epilepsy surgery and dietary treatments may improve seizure control.[5][6][6][7][8]
Types
Under the International League Against Epilepsy (ILAE) 2017 classification of the epilepsies, focal onset epilepsy occurs from seizures arising from a biological neural network within a single cerebral hemisphere.[9][10] Temporal lobe epilepsy occurs from seizures arising within the temporal lobe.[10] Temporal lobe epilepsy is the most common focal onset epilepsy, and 80% of temporal lobe epilepsy is mesial (medial) temporal lobe epilepsy, temporal lobe epilepsy arising from the inner (medial) part of the temporal lobe that may involve the hippocampus, parahippocampal gyrus or amygdala.[2][11] The less common lateral temporal lobe or neocortical temporal lobe seizures arise from the outer (lateral) temporal lobe.[2] The ILAE 2017 classification distinguishes focal aware from focal impaired seizures.[10] A focal aware temporal lobe seizure occurs if a person remains aware of what occurs during the entire seizure; awareness may be retained even if impaired responsiveness occurs during the seizure.[10] A focal impaired awareness temporal lobe seizure occurs if a person becomes unaware during any part of the seizure.[10]
Symptoms and behavior
Medial temporal lobe epilepsy
During a temporal lobe seizure, a person may experience a seizure aura; an aura is an autonomic, cognitive, emotional or sensory experience that commonly occurs during the beginning part of a seizure.[10][2] The common medial temporal lobe seizure auras include a rising epigastric feeling, abdominal discomfort, taste (gustatory), smell (olfactory), tingling (somatosensory), fear, déjà vu, jamais vu, flushing, or rapid heart rate (tachycardia).[2] A person may then stare blankly, appear motionless (behavioral arrest) and lose awareness.[2] Repeated stereotyped motor behaviors (automatisms) may occur such as repeated swallowing, lip smacking, picking, fumbling, patting or vocalizations.[2] Dystonic posture is an unnatural stiffening of one arm occurring during a seizure.[12] A dystonic posture on one side of the body commonly indicates seizure onset from the opposite side of the brain e.g. right arm dystonic posture arising from a left temporal lobe seizure.[12] Impaired language function (dysphasia) during or soon following a seizure is more likely to occur when seizures arise from the language dominant side of the brain.[12]
Lateral temporal lobe epilepsy
The common auras from seizures arising from primary auditory cortex include vertigo, humming sound, ringing sound, buzzing sound, hearing a song, hearing voices or altered hearing sensation.[2] Lateral temporal lobe seizures arising from the temporal-parietal lobe junction may cause complex visual hallucinations.[2] In comparison to mesial temporal lobe seizures, lateral temporal lobe seizures are briefer duration seizures, occur with earlier loss of awareness, and are more likely become a focal to bilateral tonic-clonic seizure.[2] Impaired language function (dysphasia) during or soon following a seizure is more likely to occur when seizures arise from the language dominant side of the brain.[12]
Comorbidities
Memory
The major cognitive impairment in mesial temporal lobe epilepsy is a progressive memory impairment.[13]:71 This involves declarative memory impairment, including episodic memory and semantic memory, and is worse when medications fail to control seizures.[14][15][13]:71 Mesial temporal lobe epilepsy arising from the language dominant hemisphere impairs verbal memory, and mesial temporal lobe epilepsy arising from the language non-dominant hemisphere impairs nonverbal memory.[13]:71[15]
Psychiatric comorbidities
Psychiatric disorders are more common among those with epilepsy, and the highest prevalence occurs among those with temporal lobe epilepsy.[16] The most common psychiatric comorbidity is major depressive disorder.[16] Other disorders include post-traumatic stress disorder, general anxiety disorder, psychosis, obsessive-compulsive disorder, schizophrenia, bipolar disorder, substance use disorder and a ~9% prevalence of suicide.[16]
Personality
Causes
Hippocampal sclerosis, brain tumor, traumatic brain injury, cerebral vascular malformation, neuronal migration disorders, infections such as encephalitis and meningitis, autoimmune disease (limbic encephalitis) and genetic disorders may cause temporal lobe epilepsy.[17]
Risk factors
Many persons with uncontrolled temporal lobe epilepsy had childhood febrile seizures.[18] A brief febrile seizure only slighty increases the risk for developing afebrile seizures.[19] However, the prolonged seizure of febrile status epilepticus leads to a 9% risk for developing epilepsy.[19] There is no clear relationship between febrile seizures and development of hippocampal sclerosis.[19]
Mechanisms
thumb|Scalp electrodes are placed to record an electroencephalogram

Neuronal loss
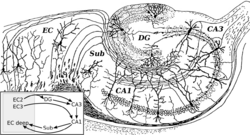
Hippocampal sclerosis occurs with severe CA1 and less severe CA3 and CA4 neuronal loss.[20] Experimental research has shown that N-methyl-d-aspartate receptor (NMDA) receptor activation causes neuronal cell loss, and electrical stimulation-induced animal models of temporal lobe epilepsy duplicate the cell loss pattern of temporal lobe epilepsy in humans.[20] Repetitive seizures irreversibly damage interneurons leading to persistent loss of recurrent inhibition.[20] Damage of GABAergic interneurons lead to loss of inhibition, uncontrolled neuronal firing, leading to seizures.[20] The secondary epileptogenesis hypothesis is that repetitive seizures lead to interneuron loss, loss of glutamatergic principal neurons, axonal sprouting, and formation of new recurrent glutamatergic excitatory circuits leading to a more severe epilepsy.[21] Mechanisms related to neuronal loss incompletely account for temporal lobe epilepsy as temporal lobe epilepsy may occur with only minimal neuronal cell loss.[20]
Neuron-specific type 2 K+/Cl− cotransporter (KCC2) mutation
This KCC2 mutation prevents subicular neurons from potassium and chloride ion extrusion, leading to intracellular chloride accumulation, and positive γ-Aminobutyric acid (GABA) mediated currents.[20] Accumulated chloride efflux through GABA receptors leads to neuronal depolarization, increased neuronal excitability and ultimately seizures.[20] Persons with this mutation have mesial temporal lobe epilepsy with hippocampal sclerosis.[20]
Granule cell dispersion
Dentate gyrus granule cell dispersion refers to a granule cell layer that is widened, poorly demarcated, or accompanied by granule cells outside the layer (ectopic granule cells).[22]:1318 In the normal brain, dentate granule cells block seizure spread from entorhinal cortex to the hippocampus.[20] A hypothesis is that granule cell dispersion may disrupt the normal mossy fiber pathway connecting granule cells and CA3 pyramidal cells leading to mossy fiber sprouting and new excitatory networks capable of generating seizures.[20] However, a study has shown that a similar pattern of granule cell dispersion may occur in persons without epilepsy.[23]
Cortical developmental malformations
Focal cortical dysplasia is a brain malformation that may cause temporal lobe epilepsy.[20] This malformation may cause abnormal cortical layers (dyslamination ), occur with abnormal neurons (dysmorphic neurons, balloon cells ) and may occur with a brain tumor or vascular malformation.[20] An abnormality of the MTOR pathway leads to hyperexcitable glutamate mediated neurons leading to seizures.[20]
Diagnosis
Electroencephalogram
The temporal lobe epileptiform discharge is a pattern seen on the electroencephalgram (EEG) test; temporal lobe epileptiform discharges occur between seizures and confirm the diagnosis of temporal lobe epilepsy.[3] Long-term video-EEG monitoring may record the behavior and EEG during a seizure.[3] Magnetoencephalography may diagnose temporal lobe epilepsy by recording epileptiform discharges or seizure patterns arising from the magnetic fields of neural electrical currents.[3]
Neuroimaging
Neuroimaging tests may identify the cause for seizures and the seizure focus, the brain location where seizures begin.[4] In newly diagnosed epilepsy, magnetic resonance imaging (MRI) can detect brain lesion in up to 12 to 14% of persons with epilepsy.[24] However, for those with chronic epilepsy, MRI can detect brain lesion in 80% of the persons with epilepsy.[24] 3-Tesla MRI scan is advised for those with evidence of focal epilepsy such as temporal lobe epilepsy.[4] Abnormalities identified by MRI scan include hippocampal sclerosis, focal cortical dysplasia, other cortical developmental brain malformations, developmental and low-grade tumors, cavernous hemangioma, hypoxic-ischemic brain injury, traumatic brain injury and encephalitis.[4]
18F-fluorodeoxyglucose (18F-FDG) brain positron emission tomography (PET) may show a brain region of decreased glucose metabolism at a time between seizures; this hypometabolic region may correspond to the seizure focus, and PET scan is more sensitive for temporal lobe seizure focus localization compared to epilepsy arising from other brain lobes.[4] Single-photon emission computed tomography (SPECT) may show a region of decreased blood flow occurring 40-60 seconds after injection during the seizure; this reduced blood flow region may correspond to the seizure focus.[4]
Computed tomography (CT) scan is less sensitive than MRI scan for identifying small tumors, vascular malformations, cortical developmental brain malformations, and abnormalities in the medial temporal lobe.[24] CT scan is advised in emergencies when the suspected cause of epilepsy may be intracerebral hemorrhage, brain abscess, large cerebral infarction or subdural empyema.[4][24] A person who requires neuroimaging but cannot have a MRI scan due to implanted devices such as a cardiac pacemaker, defibrillator or cochlear implant may receive a CT scan. CT scan may better demonstrate calcium containing brain abnormalities causing epilepsy such as in tuberous sclerosis and Sturge–Weber syndrome.[4][24]
Treatment
Medical treatment
Anticonvulsant oral medications control seizures in about two-thirds of persons with epilepsy, and control commonly occurs with one or two medications.[25]
Surgical treatment
Those with uncontrolled seizures despite treatment with multiple anticonvulsant medications have pharmacoresistant epilepsy, and they may require epilepsy surgery to achieve seizure control.[9][25]
Penfield and Flanigan first described anterior temporal lobectomy, partial surgical removal of the temporal lobe, for treatment of mesial temporal lobe epilepsy in 1950.[26] In a prospective randomized controlled trial comparing anterior temporal lobectomy to medical therapy for pharmacoresistant temporal lobe epilepsy, surgery was more effective than medical therapy with 1-year seizure free outcome occurring in 58% of persons with anterior temporal lobectomy compared to 8% of persons with drug treatment.[5] Among those with intractable mesial temporal lobe epilepsy and hippocampal sclerosis, about 70% become seizure-free after epilepsy surgery.[27]:751 Studies show that language dominant anterior temporal lobectomy may lead to verbal memory decline.[15] However, study outcomes are more variable on language non-dominant anterior temporal lobectomy leading to nonverbal memory decline.[15]
Magnetic resonance-guided laser interstitial thermal therapy, stereotactic radiosurgery, and stereotactic radiofrequency ablation are surgical methods that treat epilepsy by destroying the abnormal brain tissue that causes seizures.[28][29] [30]
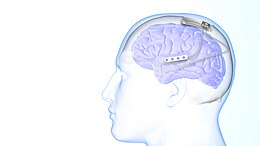
Neurostimulation may also improve seizure control.[6] The vagus nerve stimulator (VNS) is surgically implanted in the chest, and delivers programmed electrical stimulation to the vagus nerve in the neck.[31] The responsive neurostimulation device is implanted in the skull, monitors electrical brain activity for seizures, and responds to seizures with programmed electrical stimulation to one or two brain areas.[32] Programmed deep brain stimulation of the anterior thalamic nucleus may treat seizures arising from more than 2 brain areas.[6]
Dietary treatment
The ketogenic diet and modified Atkins diet are additional temporal lobe epilepsy treatment options.[7][8]
Remission
Among those who develop childhood temporal lobe epilepsy, epilepsy remits in about one-third of children.[33] Remission was more likely among those without hippocampal sclerosis, brain tumor, or focal cortical dysplasia on MRI scan.[33]
Notes
- ↑ Muhlhofer et al. 2017.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 Chowdhury et al. 2021.
- ↑ 3.0 3.1 3.2 3.3 Javidan 2012.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Duncan 2019.
- ↑ 5.0 5.1 Wiebe et al. 2001.
- ↑ 6.0 6.1 6.2 6.3 Touma et al. 2022.
- ↑ 7.0 7.1 Freeman, Kossoff & Hartman 2007.
- ↑ 8.0 8.1 Rezaei et al. 2019.
- ↑ 9.0 9.1 Scheffer et al. 2017.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Fisher et al. 2017.
- ↑ Tatum 2012.
- ↑ 12.0 12.1 12.2 12.3 Rusu et al. 2005.
- ↑ 13.0 13.1 13.2 Zeman, Kapur & Jones-Gotman 2012.
- ↑ Quiroga 2012.
- ↑ 15.0 15.1 15.2 15.3 Bauman, Devinsky & Liu 2019.
- ↑ 16.0 16.1 16.2 Lu et al. 2021.
- ↑ Vadlamudi 2003.
- ↑ Patterson, Baram & Shinnar 2014.
- ↑ 19.0 19.1 19.2 Mewasingh, Chin & Scott 2020.
- ↑ 20.00 20.01 20.02 20.03 20.04 20.05 20.06 20.07 20.08 20.09 20.10 20.11 20.12 Ong 2019.
- ↑ Ben-Ari & Dudek 2010.
- ↑ Blümcke et al. 2013.
- ↑ Roy, Millen & Kapur 2020.
- ↑ 24.0 24.1 24.2 24.3 24.4 Salmenpera & Duncan 2005.
- ↑ 25.0 25.1 Kwan & Brodie 2000.
- ↑ Penfield & Flanigan 1950.
- ↑ Lamberink et al. 2020.
- ↑ Chen et al. 2023.
- ↑ Kerezoudis et al. 2022.
- ↑ Mustafa & Zaben 2022.
- ↑ Goggins, Mitani & Tanaka 2022.
- ↑ Geller 2018.
- ↑ 33.0 33.1 Spooner et al. 2006.
References
- Arzy, S; Schurr, R (2016). ""God has sent me to you":Right temporal epilepsy, left prefrontal psychosis.". Epilepsy & Behavior 60: 7–10. doi:10.1016/j.yebeh.2016.04.022. PMID 27176877.
- Bauman, Kristie; Devinsky, Orrin; Liu, Anli A. (2019). "Temporal lobe surgery and memory: Lessons, risks, and opportunities". Epilepsy & Behavior 101 (Pt A): 106596. doi:10.1016/j.yebeh.2019.106596. PMID 31711868.
- Ben-Ari, Yehezkel; Dudek, F. Edward (2010). "Primary and Secondary Mechanisms of Epileptogenesis in the Temporal Lobe: There is a before and an After" (in en). Epilepsy Currents 10 (5): 118–125. doi:10.1111/j.1535-7511.2010.01376.x. ISSN 1535-7597. PMID 20944823.
- Blümcke, Ingmar; Thom, Maria; Aronica, Eleonora; Armstrong, Dawna D. et al. (2013). "International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: A Task Force report from the ILAE Commission on Diagnostic Methods". Epilepsia 54 (7): 1315–1329. doi:10.1111/epi.12220. PMID 23692496.
- Chen, Jia-Shu; Lamoureux, Audrey-Anne; Shlobin, Nathan A.; Elkaim, Lior M. et al. (2023). "Magnetic Resonance-guided Laser Interstitial Thermal Therapy for Drug-Resistant Epilepsy: A Systematic Review and Individual Participant Data Meta-Analysis" (in en). Epilepsia 64 (8): 1957–1974. doi:10.1111/epi.17560. ISSN 0013-9580. PMID 36824029.
- Chowdhury, Fahmida A; Silva, Rui; Whatley, Benjamin; Walker, Matthew C (2021). "Localisation in focal epilepsy: a practical guide". Practical Neurology 21 (6): 481–491. doi:10.1136/practneurol-2019-002341. PMID 34404748. https://pn.bmj.com/content/21/6/481.
- David, Daniel; Fleminger, Simon; Kopelman, Michael; Lovestone, Simon; Mellers, John (2012) (in en). Lishman's Organic Psychiatry: A Textbook of Neuropsychiatry. John Wiley & Sons. ISBN 978-0-470-67507-6. https://books.google.com/books?id=ohG6I1C_NeAC.
- d'Orsi, Giuseppe; Tinuper, Paolo (2006). ""I heard voices*": from semiology, a historical review, and a new hypothesis on the presumed epilepsy of Joan of Arc.". Epilepsy & Behavior 9 (1): 152–157. doi:10.1016/j.yebeh.2006.04.020. PMID 16750938. https://www.sciencedirect.com/science/article/pii/S1525505006001752.
- Duncan, John S. (2019). "Brain imaging in epilepsy" (in en). Practical Neurology 19 (5): 438–443. doi:10.1136/practneurol-2018-002180. ISSN 1474-7758. PMID 31420416. https://pn.bmj.com/content/19/5/438.
- Fisher, RS; Cross, JH; French, JA; Higurashi, N et al. (2017). "Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology.". Epilepsia 58 (4): 522–530. doi:10.1111/epi.13670. PMID 28276060.
- Freeman, JM; Kossoff, EH; Hartman, AL (2007). "The ketogenic diet: one decade later.". Pediatrics 119 (3): 535–43. doi:10.1542/peds.2006-2447. PMID 17332207.
- Geller, Eric B. (2018-11-01). "Responsive neurostimulation: Review of clinical trials and insights into focal epilepsy" (in English). Epilepsy & Behavior 88: 11–20. doi:10.1016/j.yebeh.2018.06.042. ISSN 1525-5050. PMID 30243756. https://pubmed.ncbi.nlm.nih.gov/30243756/.
- Goggins, Eibhlin; Mitani, Shuhei; Tanaka, Shinji (2022). "Clinical perspectives on vagus nerve stimulation: present and future". Clinical Science 136 (9): 695–709. doi:10.1042/CS20210507. PMID 35536161. PMC 9093220. https://portlandpress.com/clinsci/article/136/9/695/231280/Clinical-perspectives-on-vagus-nerve-stimulation.
- Javidan, Manouchehr (2012). "Electroencephalography in Mesial Temporal Lobe Epilepsy: A Review". Epilepsy Research and Treatment 2012: 1–17. doi:10.1155/2012/637430. PMID 22957235.
- Kerezoudis, Panagiotis; Tsayem, Idriss N.; Lundstrom, Brian N.; Van Gompel, Jamie J. (2022). "Systematic review and patient-level meta-analysis of radiofrequency ablation for medically refractory epilepsy: Implications for clinical practice and research" (in en). Seizure 102: 113–119. doi:10.1016/j.seizure.2022.10.003. ISSN 1059-1311. PMID 36219914.
- Kwan, P.; Brodie, M.J. (2000). "Early identification of refractory epilepsy". NEJM 342 (5): 314–319. doi:10.1056/NEJM200002033420503. PMID 10660394.
- Lamberink, Herm J; Otte, Willem M; Blümcke, Ingmar; Braun, Kees P J et al. (2020). "Seizure outcome and use of antiepileptic drugs after epilepsy surgery according to histopathological diagnosis: a retrospective multicentre cohort study". The Lancet Neurology 19 (9): 748–757. doi:10.1016/S1474-4422(20)30220-9. PMID 32822635. https://www.thelancet.com/article/S1474-4422(20)30220-9/fulltext.
- Lu, Elaine; Pyatka, Nataliya; Burant, Christopher J; Sajatovic, Martha (2021). "Systematic Literature Review of Psychiatric Comorbidities in Adults with Epilepsy". Journal of Clinical Neurology 17 (2): 176–186. doi:10.3988/jcn.2021.17.2.176. PMID 33835737.
- Mewasingh, Leena D; Chin, Richard F M; Scott, Rod C (2020). "Current understanding of febrile seizures and their long-term outcomes". Developmental Medicine & Child Neurology 62 (11): 1245–1249. doi:10.1111/dmcn.14642. PMID 32748466.
- Muhlhofer, Wolfgang; Tan, Yee-Leng; Mueller, Susanne G.; Knowlton, Robert (2017). "MRI negative temporal lobe epilepsy—What do we know?". Epilepsia 58 (5): 727–742. doi:10.1111/epi.13699. PMID 28266710.
- Mustafa, Mohamed; Zaben, Malik (2022). "Minimal invasive brain surgery for epilepsy; can it be the future?" (in en). Journal of Neurology 269 (11): 6178–6180. doi:10.1007/s00415-022-11360-z. ISSN 1432-1459. PMID 36098841.
- Ong, Leong Tung (2019). "Temporal Lobe Epilepsy – Pathophysiology and Mechanisms". European Neurological Review 14 (2): 66. doi:10.17925/ENR.2019.14.2.66. https://www.researchgate.net/publication/339143583.
- Patterson, Katelin P.; Baram, Tallie Z.; Shinnar, Shlomo (2014). "Origins of Temporal Lobe Epilepsy: Febrile Seizures and Febrile Status Epilepticus" (in en). Neurotherapeutics 11 (2): 242–250. doi:10.1007/s13311-014-0263-4. PMID 24604424. PMC 3996115. https://escholarship.org/content/qt2831p55r/qt2831p55r_noSplash_c910fdbb9d807e78eac06a2d1c6ffaa0.pdf.
- Penfield, Wilder; Flanigan, Herman (1950). "Surgical therapy of temporal lobe seizures". Archives of Neurology and Psychiatry 64 (4): 491–500. doi:10.1001/archneurpsyc.1950.02310280003001. PMID 14770593. https://jamanetwork.com/journals/archneurpsyc/article-abstract/651041.
- Quiroga, Rodrigo Quian (2012). "Concept cells:the building blocks of declarative memory functions". Nature Reviews Neuroscience 13 (8): 587–597. doi:10.1038/nrn3251. PMID 22760181. https://www.nature.com/articles/nrn3251.
- Rezaei, Shahabeddin; Abdurahman, Ahmed Abdulahi; Saghazadeh, Amene; Badv, Reza Shervin; Mahmoudi, Maryam (2019). "Short-term and long-term efficacy of classical ketogenic diet and modified Atkins diet in children and adolescents with epilepsy: A systematic review and meta-analysis". Nutritional Neuroscience 22 (5): 317–334. doi:10.1080/1028415X.2017.1387721. PMID 29069983. https://www.tandfonline.com/doi/abs/10.1080/1028415X.2017.1387721.
- Roy, Achira; Millen, Kathleen J.; Kapur, Raj P. (2020). "Hippocampal granule cell dispersion: a non-specific finding in pediatric patients with no history of seizures". Acta Neuropathologica Communications 8 (1): 54. doi:10.1186/s40478-020-00928-3. PMID 32317027.
- Rusu, V.; Chassoux, F.; Landre, E.; Bouilleret, V. et al. (2005). "Dystonic posturing in seizures of mesial temporal origin: Electroclinical and metabolic patterns". Neurology 65 (10): 1612–1619. doi:10.1212/01.wnl.0000184510.44808.50. PMID 16301490. https://n.neurology.org/content/65/10/1612.full.
- Salmenpera, T.M.; Duncan, J.S. (2005). "Imaging in epilepsy" (in en). Journal of Neurology, Neurosurgery & Psychiatry 76 (suppl 3): iii2–iii10. doi:10.1136/jnnp.2005.075135. ISSN 0022-3050. PMID 16107387. PMC 1765703. https://jnnp.bmj.com/content/76/suppl_3/iii2.
- Scheffer, Ingrid E.; Berkovic, Samuel; Capovilla, Giuseppe; Connolly, Mary B. et al. (2017). "ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology". Epilepsia 58 (4): 512–521. doi:10.1111/epi.13709. PMID 28276062.
- Sirven, Joseph I; Drazkowski, Joseph F; Noe, Katherine H (2007). "Seizures among public figures: lessons learned from the epilepsy of Pope Pius IX.". Mayo Clinic Proceedings 82 (12): 1535–1540. doi:10.1016/S0025-6196(11)61100-2. PMID 18053463.
- Spooner, C.G.; Berkovic, S.F.; Mitchell, L.A.; Wrennall, J.A. et al. (2006). "New-onset temporal lobe epilepsy in children: Lesion on MRI predicts poor seizure outcome". Neurology 67 (12): 2147–2153. doi:10.1212/01.wnl.0000248189.93630.4f. PMID 17082466. https://n.neurology.org/content/67/12/2147.short.
- Tatum, William O. (2012). "Mesial Temporal Lobe Epilepsy". Journal of Clinical Neurophysiology 29 (5): 356–365. doi:10.1097/WNP.0b013e31826b3ab7. PMID 23027091. https://pubmed.ncbi.nlm.nih.gov/23027091/.
- Tedrus, Glória Maria Almeida Souza; Fonseca, Lineu Corrêa; Höehr, Gabriela Chaves (2013). "Spirituality aspects in patients with epilepsy.". Seizure 23 (1): 25–8. doi:10.1016/j.seizure.2013.09.005. PMID 24094727.
- Tedrus, Glória Maria Almeida Souza; Fonseca, Lineu Corrêa; Fagundes, Tatiane Mariani; da Silva, Gabriela Leopoldino (2015). "Religiosity aspects in patients with epilepsy.". Epilepsy & Behavior 50: 67–70. doi:10.1016/j.yebeh.2015.06.003. PMID 26133113. https://www.sciencedirect.com/science/article/pii/S1525505015003327.
- Touma, Lahoud; Dansereau, Bénédicte; Chan, Alvin Y.; Jetté, Nathalie et al. (2022). "Neurostimulation in people with drug-resistant epilepsy: Systematic review and meta-analysis from the ILAE Surgical Therapies Commission". Epilepsia 63 (6): 1314–1329. doi:10.1111/epi.17243. ISSN 1528-1167. PMID 35352349. https://pubmed.ncbi.nlm.nih.gov/35352349/.
- Vadlamudi, L (2003). "Genetics of temporal lobe epilepsy". Journal of Neurology, Neurosurgery & Psychiatry 74 (10): 1359–1361. doi:10.1136/jnnp.74.10.1359. PMID 14570824. PMC 1757393. https://jnnp.bmj.com/content/74/10/1359.
- Wiebe, Samuel; Blume, Warren T.; Girvin, John P.; Eliasziw, Michael (2001). "A Randomized, Controlled Trial of Surgery for Temporal-Lobe Epilepsy". New England Journal of Medicine 345 (5): 311–318. doi:10.1056/NEJM200108023450501. PMID 11484687.
- Zeman, Adam; Kapur, Narinder; Jones-Gotman, Marilyn (2012). Epilepsy and memory (1. ed.). Oxford: Oxford Univ. Press. ISBN 978-0199580286. https://books.google.com/books?id=cwJREAAAQBAJ&dq=Epilepsy+and+memory&pg=PP1.
| Classification | |
|---|---|
| External resources |