Chemistry:Mazapertine: Difference between revisions
(over-write) |
(No difference)
|
Latest revision as of 08:35, 2 May 2023
| |
| Names | |
|---|---|
| Preferred IUPAC name
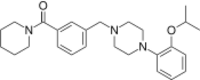
(Piperidin-1-yl){3-[(4-{2-[(propan-2-yl)oxy]phenyl}piperazin-1-yl)methyl]phenyl}methanone | |
| Other names
RWJ-37796
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C26H35N3O2 | |
| Molar mass | 421.585 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mazapertine (RWJ-37796) is an antipsychotic agent that was developed by Johnson & Johnson but never marketed. It exerts its pharmacological effect through affinity for dopamine D2, serotonin 5-HT1A, and α1-adrenergic receptors.[1]
Mazapertine is safe and well tolerated when administered orally.[2]
Analogs of mazapertine with conformational restriction have been prepared and have greater affinity for the 5-HT1A receptor.[3]
Synthesis
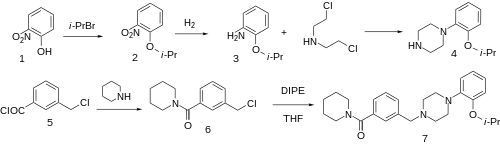
Alkylation of 2-Nitrophenol [88-75-5] (1) with isopropyl bromide gives 2-isopropoxynitrobenzene [38753-50-3] (2). Catalytic hydrogenation of nitro group gives 2-isopropoxyaniline [29026-74-2] (3). Intermolecular ring formation of this aniline with nornitrogen mustard [334-22-5] yields 1-(2-isopropoxyphenyl)piperazine [54013-91-1] (4).
Amide formation of 3-(chloromethyl)benzoyl chloride [63024-77-1] (5) with piperidine gives 1-[3-(chloromethyl)benzoyl]piperidine [148583-64-6] (6).
Ex 1: The last step is the convergent synthesis between the above two arms of the synthesis to afford the alkylation product mazapertine (7).
References
- ↑ Reitz, A. B.; Baxter, E. W.; Codd, E. E.; Davis, C. B.; Jordan, A. D.; Maryanoff, B. E.; Maryanoff, C. A.; McDonnell, M. E. et al. (1998). "Orally Active Benzamide Antipsychotic Agents with Affinity for Dopamine D2, Serotonin 5-HT1A, and Adrenergic α1Receptors". Journal of Medicinal Chemistry 41 (12): 1997–2009. doi:10.1021/jm970164z. PMID 9622541.
- ↑ Kleinbloesem, C. H.; Jaquet-Müller, F. O.; Al-Hamdan, Y.; Baldauf, C.; Gisclon, L.; Wesnes, K.; Curtin, C. R.; John Stubbs, R. et al. (1996). "Incremental dosage of the new antipsychotic mazapertine induces tolerance to cardiovascular and cognitive effects in healthy men*". Clinical Pharmacology & Therapeutics 59 (6): 675–685. doi:10.1016/S0009-9236(96)90008-9. PMID 8681493.
- ↑ Baxter, Ellen W.; Reitz, Allen B. (1997). "Hindered rotation congeners of mazapertine: High affinity ligands for the 5-HT1A receptor". Bioorganic & Medicinal Chemistry Letters 7 (7): 763. doi:10.1016/S0960-894X(97)00074-7.
- ↑ Reitz, Allen B.; Bennett, Debra J.; Blum, Paul S.; Codd, Ellen E.; Maryanoff, Cynthia A.; Ortegon, Marta E.; Renzi, Michael J.; Scott, Malcolm K.; Shank, Richard P.; Vaught, Jeffry L. (1994). "A New Arylpiperazine Antipsychotic with High D2/D3/5-HT1A/.alpha.1A-Adrenergic Affinity and a Low Potential for Extrapyramidal Effects". Journal of Medicinal Chemistry 37 (8): 1060–1062. doi:10.1021/jm00034a003.
- ↑ Reitz, Allen B.; Baxter, Ellen W.; Codd, Ellen E.; Davis, Coralie B.; Jordan, Alfonzo D.; Maryanoff, Bruce E.; Maryanoff, Cynthia A.; McDonnell, Mark E.; Powell, Eugene T.; Renzi, Michael J.; Schott, Mary R.; Scott, Malcolm K.; Shank, Richard P.; Vaught, Jeffry L. (1998). "Orally Active Benzamide Antipsychotic Agents with Affinity for Dopamine D2, Serotonin 5-HT1A, and Adrenergic α1Receptors". Journal of Medicinal Chemistry. 41 (12): 1997–2009. doi:10.1021/jm970164z.
- ↑ Allen B. Reitz, U.S. Patent 5,569,659 (1996 to Ortho McNeil Pharmaceutical Inc).
 |