Chemistry:Loxapine
Loxapine, sold under the brand names Loxitane and Adasuve (inhalation only) among others, is a tricyclic[1] antipsychotic medication used primarily in the treatment of schizophrenia. The medicine is a member of the dibenzoxazepine class and structurally very similar to clozapine. Several researchers have argued that loxapine, initially classified as a typical antipsychotic, behaves as an atypical antipsychotic.[2]
Loxapine may be metabolized by N-demethylation to amoxapine, a tricyclic antidepressant.[3]
Medical uses
The US Food and Drug Administration (FDA) has approved loxapine inhalation powder for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults.[4]
A brief review of loxapine found no conclusive evidence that it was particularly effective in patients with schizophrenia.[5] A subsequent systematic review considered that the limited evidence did not indicate a clear difference in its effects from other antipsychotics.[6]
Available forms
Loxapine can be taken by mouth.[7] It is also available as an intramuscular injection and as a powder for inhalation.[4][7]
Side effects
Loxapine can cause side effects that are generally similar to that of other antipsychotic medications. These include, e.g., gastrointestinal problems (like constipation and abdominal pain), cardiovascular problems (like tachycardia), moderate likelihood of drowsiness (relative to other antipsychotics),[8] and movement problems (i.e. extrapyramidal symptoms [EPS]).[9] At lower dosages its propensity for causing EPS appears to be similar to that of atypical antipsychotics.[10] Although it is structurally similar to clozapine, it has much lower risk of agranulocytosis (which, even with clozapine, is 0.8%); however, mild and temporary fluctuations in blood leukocyte levels can occur.[11][12] Abuse of loxapine has been reported.[13]
The inhaled formulation of loxapine carries a low risk for a type of airway adverse reaction called bronchospasm that is not thought to occur when loxapine is taken by mouth.[4]
Pharmacology
Mechanism of action
Some scientists say loxapine is a "mid-potency" typical antipsychotic.[12] However, unlike most other typical antipsychotics, it has significant potency at the 5HT2A receptor (6.6 nM), which is similar to atypical antipsychotics like clozapine (5.35 nM). The higher likelihood of EPS with loxapine, compared to clozapine, may be due to its higher affinity for the D2 receptor compared to clozapine, which has one of the lowest binding affinities at the D2 receptor of any antipsychotic.[12]
| Site | LOX | AMX |
|---|---|---|
| 5-HT1A | 2460 | ND |
| 5-HT1B | 388 | ND |
| 5-HT1D | 3470 | ND |
| 5-HT1E | 1400 | ND |
| 5-HT2A | 6.6 | 0.5 |
| 5-HT2C | 13 | 2 (rat) |
| 5-HT3 | 190 | ND |
| 5-HT5A | 780 | ND |
| 5-HT6 | 31 | 50 |
| 5-HT7 | 88 | 40 (rat) |
| α1A | 31 | ND |
| α1B | 53 | ND |
| α2A | 151 | ND |
| α2B | 108 | ND |
| α2C | 80 | ND |
| β1 | 10000+ | ND |
| β2 | 10000+ | ND |
| M1 | 120 | ND |
| M2 | 445 | ND |
| M3 | 211 | ND |
| M4 | 1270 | ND |
| M5 | 166 | ND |
| D1 | 54 | ND |
| D2 | 11 | 21 |
| D3 | 19 | 21 |
| D4 | 8.4 | 21 |
| D5 | 75 | ND |
| H1 | 2.2–4.9 | 7.9–25 |
| H2 | 208 | ND |
| H3 | 55000 | >100,000 |
| H4 | 5050–8710 | 6,310 |
| SERT | 10000+ | 58 |
| NET | 5700 | 16 |
| DAT | 10000+ | 58 |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | ||
Use as an Inhaled Agitation Medication
Loxapine has a relatively strong safety profile compared to similar antipsychotic drugs, such as quetiapine, olanzapine, and clozapine, with a relatively lower incidence of metabolic side-effects (although higher extrapyramial side-effects).[14] Given its strong safety profile and speed/efficacy when given intramuscularly for acute agitation,[15] an inhaled version of the drug (Adasuve) has been developed to be applied nasally for agitated patients needing to be rapidly de-escalated.[16]
Pharmacokinetics
Loxapine is metabolized to amoxapine, as well as its 8-hydroxy metabolite (8-hydroxyloxapine).[17] Amoxapine is further metabolized to its 8-hydroxy metabolite (8-hydroxyamoxapine), which is also found in the blood of people taking loxapine.[18] At steady-state after taking loxapine by mouth, the relative amounts of loxapine and its metabolites in the blood is as follows: 8-hydroxyloxapine > 8-hydroxyamoxapine > loxapine.[18]
The pharmacokinetics of loxapine change depending on how it is given. Intramuscular injections of loxapine lead to higher blood levels and area under the curve of loxapine than when it is taken by mouth.[18]
Chemistry
Loxapine is a dibenzoxazepine and is structurally very similar to clozapine, an atypical antipsychotic.
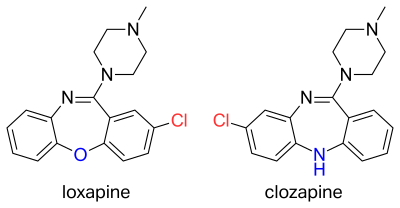
References
- ↑ "Revisiting loxapine: a systematic review". Annals of General Psychiatry 14. 2015-04-01. doi:10.1186/s12991-015-0053-3. PMID 25859275.
- ↑ "Does loxapine have "atypical" properties? Clinical evidence". The Journal of Clinical Psychiatry 60 Suppl 10 (Suppl 10): 42–46. 1999. PMID 10340686. https://www.psychiatrist.com/jcp/psychopharmacology/does-loxapine-atypical-properties-clinical-evidence/.
- ↑ "Simultaneous quantitation of loxapine, amoxapine and their 7- and 8-hydroxy metabolites in plasma by high-performance liquid chromatography". Journal of Chromatography 564 (1): 213–221. March 1991. doi:10.1016/0378-4347(91)80083-O. PMID 1860915.
- ↑ 4.0 4.1 4.2 "ADASUVE Package Insert". Galen US Inc. https://www.adasuve.com/PDF/AdasuvePI.pdf.
- ↑ "Clozapine and loxapine for schizophrenia". Drug and Therapeutics Bulletin 29 (11): 41–42. May 1991. doi:10.1136/dtb.29.11.41. PMID 1747161.
- ↑ "Loxapine for schizophrenia". The Cochrane Database of Systematic Reviews 2007 (4). October 2007. doi:10.1002/14651858.CD001943.pub2. PMID 17943763.
- ↑ 7.0 7.1 "LOXITANE Package Insert". Watson Laboratories, Inc.. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/017525s051,017658s038,018039s024lbl.pdf.
- ↑ The Maudsley prescribing guidelines in psychiatry (11th ed.). Chichester, West Sussex: Wiley-Blackwell. 2012. ISBN 978-0-470-97948-8.
- ↑ "Loxapine for schizophrenia". The Cochrane Database of Systematic Reviews 2007 (4). October 2007. doi:10.1002/14651858.CD001943.pub2. PMID 17943763.
- ↑ "Inhaled loxapine for rapid treatment of agitation in schizophrenia and bipolar disorder: An update". Neuropsychiatry 2 (3): 253–260. 2012. doi:10.2217/npy.12.23.
- ↑ "Loxapine: fifteen years' clinical experience". Psychosomatics 23 (3): 261–271. March 1982. doi:10.1016/S0033-3182(82)73416-4. PMID 7041162.
- ↑ 12.0 12.1 12.2 "A neurochemical basis for the antipsychotic activity of loxapine: interactions with dopamine D1, D2, D4 and serotonin 5-HT2 receptor subtypes". Journal of Psychiatry & Neuroscience 21 (1): 29–35. January 1996. PMID 8580115.
- ↑ "Loxapine abuse". The New England Journal of Medicine 310 (9): 598. March 1984. doi:10.1056/NEJM198403013100920. PMID 6694719.
- ↑ "Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis" (in English). Lancet (London, England) 394 (10202): 939–951. 2019-09-14. doi:10.1016/S0140-6736(19)31135-3. ISSN 0140-6736. PMID 31303314.
- ↑ "Efficacy and Safety of Loxapine in Acute Agitation: A Systematic Review of Interventional Studies". The Primary Care Companion for CNS Disorders 25 (6). 2023-12-19. doi:10.4088/PCC.23r03552. ISSN 2155-7780. PMID 38134395. https://www.psychiatrist.com/pcc/efficacy-safety-loxapine-acute-agitation-systematic-review-interventional-studies/.
- ↑ "Revisiting loxapine: a systematic review". Annals of General Psychiatry 14. 2015. doi:10.1186/s12991-015-0053-3. ISSN 1744-859X. PMID 25859275.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedDrugPoint - ↑ 18.0 18.1 18.2 "Clinical and plasma level characteristics of intramuscular and oral loxapine". Psychopharmacology 56 (2): 225–232. March 1978. doi:10.1007/BF00431855. PMID 417377.
External links
- "Loxapine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/loxapine.
 |
