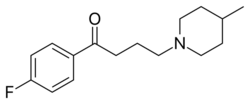
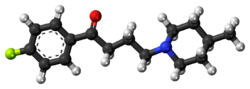
Chemistry:Melperone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Buronil |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, intramuscular injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 87% (IM), 54% (Oral via syrup), 65% (Oral, tablet)[1] |
| Protein binding | 50% |
| Metabolism | Hepatic |
| Elimination half-life | 3–4 hours (oral)[1] 6 hours (IM) |
| Excretion | Renal (70% as metabolites, 5.5–10.4% as unchanged drug)[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H22FNO |
| Molar mass | 263.356 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Melperone (Bunil (PT), Buronil (AT, BE, CZ, Denmark , Finland †, NL†, Norway †, SE), Eunerpan (Germany ))[3] is an atypical antipsychotic of the butyrophenone chemical class, making it structurally related to the typical antipsychotic haloperidol. It first entered clinical use in 1960s.[4]
Marketing and indications
It has been tried in treatment-resistant cases of schizophrenia with some (albeit limited) success.[4][5][6][7] It has also been reported effective in the treatment of L-DOPA and other forms of psychosis in Parkinson's disease[8] (although a multicentre, double-blind, placebo-controlled study conducted in 2012 failed to support these findings[9]). It is also known to possess anxiolytic properties.[10] It is marketed in the following countries:[3][11]
- Austria
- Belgium
- Czech Republic
- Denmark
- Estonia
- Finland
- Germany
- Iceland
- Lithuania
- Latvia
- Portugal
- Sweden
Adverse effects
Melperone is reported to produce significantly less weight gain than clozapine and approximately as much weight gain as typical antipsychotics.[12] It is also purported to produce around as much prolactin secretion as clozapine (which is virtually nil).[13] It is also purported to produce sedative effects[14] and QT interval prolongation.[15] It is also known to produce less extrapyramidal side effects than the first-generation (typical) antipsychotic, thiothixene.[16] It can also produce (usually relatively mild) dry mouth.[17]
- Constipation
- Diarrhea
- Nausea
- Vomiting
- Appetite loss
- Hypersalivation (drooling)
- Extrapyramidal side effects (e.g. tremor, dystonia, hypokinesis, akathisia, dyskinesias)
- Insomnia
- Agitation
- Headache
- Dizziness
- Fatigue
- Miosis
- Mydriasis
- Blurred vision
- Elevated liver enzymes (esp. ALT and GGTP)
- Tardive dyskinesia
- Neuroleptic malignant syndrome
- Blood dyscrasias (pancytopenia, agranulocytosis, leukopenia, thrombocytopenia, etc.)
- Seizures (probably rare/uncommon)
- Increased intraocular pressure
- Intrahepatic cholestasis (probably rare)
- Orthostatic hypotension (probably common)
- Arrhythmias
- Rash
- Hyperprolactinemia (which can lead to e.g. galactorrhea, gynecomastia)
- Weight gain
- Increased appetite
Interactions
Melperone is reported to be a CYP2D6 inhibitor.[20][21][22]
Pharmacology
Melperone binds to the dopamine D2 receptor, just like all other clinically-utilized antipsychotics, but it does so with a very low affinity and hence may be liable to rapidly dissociate from the D2 receptor hence potentially giving it the profile of an atypical antipsychotic.[23]
| Receptor | Ki [nM][24] |
|---|---|
| 5-HT1A | 2,200 |
| 5-HT1D | 3,400 |
| 5-HT2A | 230 |
| 5-HT2C | 2,100 |
| 5-HT6 | 1,254 |
| 5-HT7 | 578 |
| α1 | 180 |
| α2 | 150 |
| M1 | >10,000 |
| M2 | 2,400 |
| M3 | >10,000 |
| M4 | 4,400 |
| M5 | >10,000 |
| D2 | 194 |
| D3 | 347 |
| D4 | 555 |
| H1 | 580 |
Synthesis
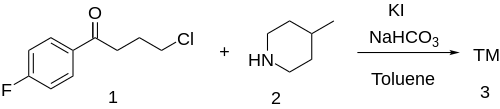
For the last step of the synthesis the sidechain 4-Chloro-4'-Fluorobutyrophenone [3874-54-2] (1) is attached to 4-Methylpiperidine (4-Pipecoline) [626-58-4] (2).
See also
References
- ↑ 1.0 1.1 1.2 "Pharmacokinetics of parenteral and oral melperone in man". European Journal of Clinical Pharmacology 23 (2): 173–6. 1982. doi:10.1007/BF00545974. PMID 7140807.
- ↑ 2.0 2.1 2.2 2.3 Product Information: Eunerpan(R), Melperonhydrochlorid (Report). Knoll Deutschland GmbH, Ludwigshafen. 1995.
- ↑ 3.0 3.1 Melperone Hydrochloride. The Royal Pharmaceutical Society of Great Britain. 30 January 2013. http://www.medicinescomplete.com/mc/martindale/current/11022-r.htm. Retrieved 3 November 2013.
- ↑ 4.0 4.1 "Auditing clinical outcomes after introducing off-licence prescribing of atypical antipsychotic melperone for patients with treatment refractory schizophrenia". TheScientificWorldJournal 2012: 512047. 2012. doi:10.1100/2012/512047. PMID 22566771.
- ↑ "Melperone in treatment-refractory schizophrenia: a case series". Therapeutic Advances in Psychopharmacology 1 (1): 19–23. February 2011. doi:10.1177/2045125311399800. PMID 23983923.
- ↑ "Melperone in the treatment of neuroleptic-resistant schizophrenia". Psychiatry Research 105 (3): 201–9. December 2001. doi:10.1016/s0165-1781(01)00346-8. PMID 11814539.
- ↑ "Melperone, an atypical antipsychotic drug, in the treatment of schizophrenia: dose-response analysis on effectiveness and tolerability, and efficacy for treatment-resistant schizophrenia and cognitive function". International Clinical Psychopharmacology 19 (3): 184. 2004. doi:10.1097/00004850-200405000-00039.
- ↑ Barbato L, Monge A, Stocchi F, Nordera G. Melperone in the treatment of iatrogenic psychosis in Parkinson’s disease. Funct Neurol. 1996 Aug;11(4):201–7.
- ↑ "Melperone is ineffective in treating Parkinson's disease psychosis". Movement Disorders 27 (6): 803–4. May 2012. doi:10.1002/mds.24942. PMID 22362330.
- ↑ "Melperone in low doses in anxious neurotic patients. A double-blind placebo-controlled clinical study". Neuropsychobiology 11 (3): 181–6. 1984. doi:10.1159/000118074. PMID 6147789.
- ↑ "Buronil generic. Price of buronil. Uses, Dosage, Side effects". https://www.ndrugs.com/?s=buronil.
- ↑ "Changes in weight and body mass index during treatment with melperone, clozapine and typical neuroleptics". Psychiatry Research 176 (2–3): 114–9. April 2010. doi:10.1016/j.psychres.2009.03.026. PMID 20199813.
- ↑ "Melperone, an aytpical antipsychotic drug with clozapine-like effect on plasma prolactin: contrast with typical neuroleptics". Human Psychopharmacology 24 (5): 415–22. July 2009. doi:10.1002/hup.1036. PMID 19551763.
- ↑ "Sedative effects and prolactin response to single oral doses of melperone". Psychopharmacology 79 (2–3): 142–7. 1983. doi:10.1007/bf00427801. PMID 6133301.
- ↑ "Melperone: electrophysiologic and antiarrhythmic activity in humans". Journal of Cardiovascular Pharmacology 15 (1): 144–9. January 1990. doi:10.1097/00005344-199001000-00023. PMID 1688972.
- ↑ "Melperone in the treatment of schizophrenia". Acta Psychiatrica Scandinavica. Supplementum 352: 35–9. 1989. doi:10.1111/j.1600-0447.1989.tb06434.x. PMID 2479227.
- ↑ "Effect of single oral doses of various neuroleptic drugs on salivary secretion rate, pH, and buffer capacity in healthy subjects". Psychopharmacology 75 (2): 114–8. 1981. doi:10.1007/bf00432171. PMID 6119724.
- ↑ 18.0 18.1 18.2 "Additional studies on side effects of melperone in long-term therapy for 1 to 15 years in psychiatric patients". Arzneimittel-Forschung 31 (4): 737–40. 1981. PMID 6113835.
- ↑ 19.0 19.1 19.2 "Additional studies on side effects of melperone in long-term therapy for 1-20 years in psychiatric patients". Arzneimittel-Forschung 36 (5): 855–60. May 1986. PMID 2873821.
- ↑ "Successful treatment of schizophrenia with melperone augmentation in a patient with phenotypic CYP2D6 ultrarapid metabolization: a case report". Journal of Medical Case Reports 6 (1): 49. February 2012. doi:10.1186/1752-1947-6-49. PMID 22309430.
- ↑ "Cytochrome P450 2D6 dependent metabolization of risperidone is inhibited by melperone". European Journal of Clinical Pharmacology 62 (4): 333–4. April 2006. doi:10.1007/s00228-006-0098-y. PMID 16534635.
- ↑ "Melperone is an inhibitor of the CYP2D6 catalyzed O-demethylation of venlafaxine". Pharmacopsychiatry 36 (1): 3–6. January 2003. doi:10.1055/s-2003-38084. PMID 12649767.
- ↑ "Atypical Antipsychotics: Mechanism of Action". FOCUS: The Journal of Lifelong Learning in Psychiatry 2 (1): 48–58. January 2004. doi:10.1176/foc.2.1.48. PMID 11873706. http://psychiatryonline.org/data/Journals/FOCUS/2601/48.pdf.
- ↑ "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. http://pdsp.med.unc.edu/pdsp.php.
- ↑ J Lassen, S Hernestam, N Sterner, U.S. Patent 3,816,433 (1974 to Ferrosan Ab).
- ↑ BE651144 idem Erik Harry Hernestam Sven, et al. GB patent 1029220 (1966 to Ferrosan); CA, 63, 13244c
- ↑ Leyva-Pérez, Antonio; Cabrero-Antonino, Jose R.; Rubio-Marqués, Paula; Al-Resayes, Saud I.; Corma, Avelino (2014). "Synthesis of the ortho/meta/para Isomers of Relevant Pharmaceutical Compounds by Coupling a Sonogashira Reaction with a Regioselective Hydration". ACS Catalysis. 4 (3): 722–731. doi:10.1021/cs401075z.
External links
- PubChem Substance
- Clinical trial number NCT00125138 for "Melperone (an Anti-Psychotic) in Patients With Psychosis Associated With Parkinson's Disease" at ClinicalTrials.gov
 |

