Chemistry:Trichloronitrosomethane
From HandWiki
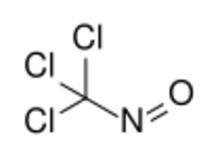
| |
| Names | |
|---|---|
| Other names
TL-358
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| CCl3NO | |
| Molar mass | 148.37 g·mol−1 |
| Appearance | Deep blue liquid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trichloronitrosomethane is a chlorinated nitrosoalkane. It is a deep blue liquid with powerful lachrymatory effects.[1]
Synthesis
Trichloronitrosomethane can be produced with following methods:[1][2]
- Oxidation of trichloromethylsulfinic acid with nitric acid.
- Reaction of sodium trichloromethylsulfinate with sodium nitrite and sodium nitrate or potassium nitrate in sulfuric acid.
- Pyrolysis of trichloroacethydroxamic acid.
Chemistry
Trichloronitrosomethane is an unstable substance. It slowly decomposes into nitrosyl chloride, nitrogen oxides, and chloropicrin over time.[1]
Trichloronitrosomethane can be reduced to phosgene oxime by hydrogen sulfide.[1]
See also
References
- ↑ 1.0 1.1 1.2 1.3 Sartori, Mario (1939). The War Gases. http://www.sciencemadness.org/library/books/the_war_gases.pdf.
- ↑ Sutcliffe, H. (September 1965). "The Synthesis of Trichloronitrosomethane". The Journal of Organic Chemistry 30 (9): 3221–3222. doi:10.1021/jo01020a516.
 |

