Chemistry:Fluoroethyl fluoroacetate
From HandWiki
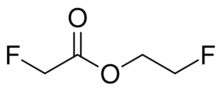
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Fluoroethyl fluoroacetate | |
| Other names
TL-855
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H6F2O2 | |
| Molar mass | 124.087 g·mol−1 |
| Appearance | Liquid |
| Hazards | |
| Main hazards | Extremely toxic |
| Lethal dose or concentration (LD, LC): | |
LDLo (lowest published)
|
1 mg/kg (oral, rats)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fluoroethyl fluoroacetate is the fluoroacetate ester of 2-fluoroethanol. It is two times more toxic than methyl fluoroacetate.[2]
See also
References
- ↑ Kitano, Hisao; Fukui, Ken-ichi; Nozu, Ryuzaburo; Osaka, Taichiro (1955). "(85) Some Reactions of Monofluoroacetic Acid Derivatives." (in Japanese). The Journal of the Society of Chemical Industry, Japan 58 (3): 224–226. doi:10.1246/nikkashi1898.58.224.
- ↑ Saunders, B. C.; Stacey, G. J. (1949). "196. Toxic fluorine compounds containing the C–F link. Part IV. (a) 2-Fluoroethyl fluoroacetate and allied compounds. (b) 2 : 2′-Difluorodiethyl ethylene dithioglycol ether". J. Chem. Soc.: 916–919. doi:10.1039/JR9490000916.
 |

