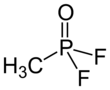
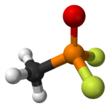
Chemistry:Methylphosphonyl difluoride
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methylphosphonic difluoride | |||
| Other names
Methylphosphonyl difluoride
Methylphosphonoyl difluoride, EA-1251 | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 4-04-00-03508 | |||
| ChemSpider | |||
| MeSH | difluoride Methylphosphonic difluoride | ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| CH3POF2 | |||
| Molar mass | 100.00 | ||
| Appearance | Colorless liquid | ||
| Odor | Pungent, acid-like[1] | ||
| Density | 1.359 g/mL (77°F)[1] | ||
| Melting point | −37 °C; −35 °F; 236 K | ||
| Boiling point | 100 °C; 212 °F; 373 K | ||
| Decomposes[1] | |||
| Vapor pressure | 36 mmHg (77°F)[1] | ||
| Hazards | |||
| Main hazards | Corrosive, toxic | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Methylphosphonyl difluoride (DF), also known as EA-1251[2] or difluoro,[3] is a chemical weapon precursor. Its chemical formula is CH3POF2. It is a Schedule 1 substance under the Chemical Weapons Convention. It is used for production of sarin and soman as a component of binary chemical weapons; an example is the M687 artillery shell, where it is used together with a mixture of isopropyl alcohol and isopropyl amine, producing sarin.
Preparation
Methylphosphonyl difluoride can be prepared by reacting methylphosphonyl dichloride with hydrogen fluoride (HF) or sodium fluoride (NaF).
Safety
Methylphosphonyl difluoride is both reactive and corrosive. It is absorbed through skin and causes burns and mild nerve agent symptoms. It reacts with water, producing HF fumes and methylphosphonic acid as a result. It is also capable of corroding glass.
Significance in international relations
In 2013–2014, the stockpile of chemicals covered by the CWC was removed from Syria and destroyed. Of the stockpile, 581 tons (over 96%) of the stockpile was DF. It was destroyed by the U.S. Army on the MV Cape Ray by hydrolysis.[4][5]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Handbook of chemical and biological warfare agents (2nd ed.). CRC Press. 24 August 2007. ISBN 9780849314346.
- ↑ "Physical properties of standard agents, candidate agents, and related compounds at several temperatures". http://apps.dtic.mil/dtic/tr/fulltext/u2/c033491.pdf.
- ↑ U. S. Army (12 Dec 1990). Potential Military Chemical/Biological Agents and Compounds. Washington, D.C.: Headquarters, Dept. of the Army. https://www.globalsecurity.org/wmd/library/policy/army/fm/3-9/fm3-9.pdf. Retrieved 12 May 2020.
- ↑ Organization for the Prohibition of Chemical Weapons (OPCW). "U.S. Completes Destruction of Sarin Precursors from Syria on the Cape Ray". OPCW News. https://www.opcw.org/media-centre/news/2014/08/us-completes-destruction-sarin-precursors-syria-cape-ray.
- ↑ Trapp, Dr. Ralf. "Lessons Learned from the OPCW Mission in Syria". OPCW. https://www.opcw.org/sites/default/files/documents/PDF/Lessons_learned_from_the_OPCW_Mission_in_Syria.pdf.
 |