Chemistry:Disulfur decafluoride
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Disulfur decafluoride | |||
| Systematic IUPAC name
Decafluoro-1λ6,2λ6-disulfane | |||
| Other names
Sulfur pentafluoride
TL-70 Agent Z | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
| MeSH | Disulfur+decafluoride | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3287 | ||
| |||
| |||
| Properties | |||
| S 2F 10 | |||
| Molar mass | 254.10 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Odor | like sulfur dioxide[1] | ||
| Density | 2.08 g/cm3 | ||
| Melting point | −53 °C (−63 °F; 220 K) | ||
| Boiling point | 30.1691 °C (86.3044 °F; 303.3191 K) | ||
| insoluble[2] | |||
| Vapor pressure | 561 mmHg (20 °C)[1] | ||
| Hazards | |||
| Main hazards | Poisonous | ||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
2000 mg/m3 (rat, 10 min) 1000 mg/m3 (mouse, 10 min) 4000 mg/m3 (rabbit, 10 min) 4000 mg/m3 (guinea pig, 10 min) 4000 mg/m3 (dog, 10 min)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 0.025 ppm (0.25 mg/m3)[1] | ||
REL (Recommended)
|
C 0.01 ppm (0.1 mg/m3)[1] | ||
IDLH (Immediate danger)
|
1 ppm[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
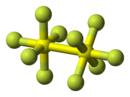
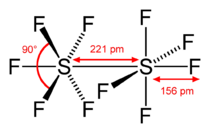
Disulfur decafluoride is a chemical compound with the formula S
2F
10. It was discovered in 1934 by Denbigh and Whytlaw-Gray.[4] Each sulfur atom of the S
2F
10 molecule is octahedral, and surrounded by five fluorine atoms[5] and one sulfur atom. The two sulfur atoms are connected by a single bond. In the S
2F
10 molecule, the oxidation state of each sulfur atoms is +5, but their valency is 6 (they are hexavalent). S
2F
10 is highly toxic, with toxicity four times that of phosgene.
It is a colorless liquid with a burnt match smell similar to sulfur dioxide.[1]
Production
Disulfur decafluoride is produced by photolysis of SF
5Br,[6] or SF
5Cl in H2:[7]
- 2 SF
5Br → S
2F
10 + Br
2 - 2 SF
5Cl + 2 H
2 → S
2F
10 + HCl
Disulfur decafluoride arises by the decomposition of sulfur hexafluoride. It is produced by the electrical decomposition of sulfur hexafluoride (SF
6)—an essentially inert insulator used in high voltage systems such as transmission lines, substations and switchgear. S
2F
10 is also made during the production of SF
6.
Properties
The S-S bond dissociation energy is 305 ± 21 kJ/mol, about 80 kJ/mol stronger than the S-S bond in diphenyldisulfide.
At temperatures above 150 °C, S2F10 decomposes slowly (disproportionation) into SF6 and SF4:
S2F10 reacts with N2F4 to give SF5NF2. It reacts with SO2 to form SF5OSO2F in the presence of ultraviolet radiation.
- S2F10 + N2F4 → 2 SF5NF2
In the presence of excess chlorine gas, S2F10 reacts to form sulfur chloride pentafluoride (SF5Cl):
- S2F10 + Cl2 → 2 SF5Cl
The analogous reaction with bromine is reversible and yields SF5Br.[8] The reversibility of this reaction can be used to synthesize S2F10 from SF5Br.[9]
Ammonia is oxidised by S2F10 into NSF3.[10]
Toxicity
S2F10 was considered a potential chemical warfare pulmonary agent in World War II because it does not produce lacrimation or skin irritation, thus providing little warning of exposure. Disulfur decafluoride is a colorless gas or liquid with a SO2-like odor.[11] It is about four times as poisonous as phosgene. Its toxicity is thought to be caused by its disproportionation in the lungs into SF6, which is inert, and SF4, which reacts with moisture to form sulfurous acid and hydrofluoric acid.[12]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 NIOSH Pocket Guide to Chemical Hazards. "#0579". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0579.html.
- ↑ "Disulphur Decafluoride | 5714-22-7". http://www.chemicalbook.com/ChemicalProductProperty_EN_CB0751782.htm.
- ↑ "Sulfur pentafluoride". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/5714227.html.
- ↑ Denbigh, K. G.; Whytlaw-Gray, R. (1934). "The Preparation and Properties of Disulphur Decafluoride". Journal of the Chemical Society 1934: 1346–1352. doi:10.1039/JR9340001346.
- ↑ Harvey, R. B.; Bauer, S. H. (June 1953). "An Electron Diffraction Study of Disulfur Decafluoride". Journal of the American Chemical Society 75 (12): 2840–2846. doi:10.1021/ja01108a015.
- ↑ Winter, R.; Nixon, P.G.; Gard, G.L. (1998). "A new preparation of disulfur decafluoride". Journal of Fluorine Chemistry 87 (1): 85–86. doi:10.1016/S0022-1139(97)00096-1. Bibcode: 1998JFluC..87...85W.
- ↑ Savoie, Paul R.; Welch, John T. (2015). "Preparation and utility of organic pentafluorosulfanyl-containing compounds". Chemical Reviews 115. doi:10.1021/cr500336u.
- ↑ Cohen, B.; MacDiarmid, A. G. (December 1965). "Chemical Properties of Disulfur Decafluoride". Inorganic Chemistry 4 (12): 1782–1785. doi:10.1021/ic50034a025.
- ↑ Winter, R.; Nixon, P.; Gard, G. (January 1998). "A new preparation of disulfur decafluoride". Journal of Fluorine Chemistry 87 (1): 85–86. doi:10.1016/S0022-1139(97)00096-1. Bibcode: 1998JFluC..87...85W.
- ↑ Mitchell, S. (1996). Biological Interactions of Sulfur Compounds. CRC Press. p. 14. ISBN 978-0-7484-0245-8.
- ↑ "Sulfur Pentaflu". 1988 OSHA PEL Project. CDC NIOSH. 28 February 2020. https://www.cdc.gov/niosh/pel88/5714-22.html.
- ↑ Johnston, H. (2003). A Bridge not Attacked: Chemical Warfare Civilian Research During World War II. World Scientific. pp. 33–36. ISBN 978-981-238-153-8. https://archive.org/details/bridgenotattacke00john.
- Christophorou, L. G.; Sauers, I. (1991). Gaseous Dielectrics VI. Plenum Press. ISBN 978-0-306-43894-3.
- "Preparation of disulfur decafluoride" US patent 02840557, issued 24 June 1958
 |