Chemistry:Erythrose
From HandWiki
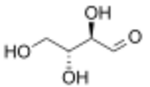 D-Erythrose
| |
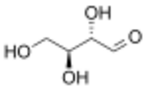 L-Erythrose
| |
| Names | |
|---|---|
| IUPAC names
(2R,3R)-2,3,4-Trihydroxybutanal (D)
(2S,3S)-2,3,4-Trihydroxybutanal (L) | |
| Identifiers | |
3D model (JSmol)
|
|
| 5805561 | |
| ChEBI | |
| ChemSpider |
|
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C4H8O4 | |
| Molar mass | 120.104 g·mol−1 |
| Appearance | Light yellow syrup |
| highly soluble | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Erythrose is a tetrose saccharide with the chemical formula C4H8O4. It has one aldehyde group, and is thus part of the aldose family. The natural isomer is D-erythrose; it is a diastereomer of D-threose.[2]
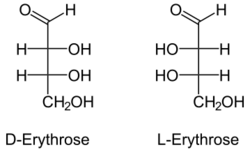
Fischer projections depicting the two enantiomers of erythrose
Erythrose was first isolated in 1849 from rhubarb by the French pharmacist Louis Feux Joseph Garot (1798-1869),[3] and was named as such because of its red hue in the presence of alkali metals (ἐρυθρός, "red").[4][5]
Erythrose 4-phosphate is an intermediate in the pentose phosphate pathway[6] and the Calvin cycle.[7]
Oxidative bacteria can be made to use erythrose as its sole energy source.[8]
See also
References
- ↑ Merck Index, 11th Edition, 3637
- ↑ "4.5: Diastereomers" (in en). 2015-04-01. https://chem.libretexts.org/Courses/University_of_Illinois_Springfield/UIS%3A_CHE_267_-_Organic_Chemistry_I_(Morsch)/Chapters/Chapter_05%3A_Stereochemistry/4.05%3A_Diastereomers.
- ↑ Obituary of Garot (1869) Journal de pharmacie et de chimie, 4th series, 9 : 472-473.
- ↑ Garot (1850) "De la matière colorante rouge des rhubarbes exotiques et indigènes et de son application (comme matière colorante) aux arts et à la pharmacie" (On the red coloring material of exotic and indigenous rhubarb and on its application (as a coloring material) in the arts and in pharmacy), Journal de Pharmacie et de Chimie, 3rd series, 17 : 5-19. Erythrose is named on p. 10: "Celui que je propose, sans y attacher toutefois la moindre importance, est celui d'érythrose, du verbe grec 'ερυθραινω, rougir (1)." (The one [i.e., name] that I propose, without attaching any importance to it, is that of erythrose, from the Greek verb ερυθραινω, to redden (1).)
- ↑ Wells, David Ames; Cross, Charles Robert; Bliss, George; Trowbridge, John; Nichols, William Ripley; Kneeland, Samuel (1851). Annual of Scientific Discovery. Boston: Gould, Kendall, and Lincoln. p. 211. https://archive.org/details/annualscientifi29crosgoog. Retrieved 11 December 2014. "erythrose discovery."
- ↑ Kruger, Nicholas J; von Schaewen, Antje (June 2003). "The oxidative pentose phosphate pathway: structure and organisation". Current Opinion in Plant Biology 6 (3): 236–246. doi:10.1016/S1369-5266(03)00039-6. PMID 12753973.
- ↑ Schwender, Jörg; Goffman, Fernando; Ohlrogge, John B.; Shachar-Hill, Yair (9 December 2004). "Rubisco without the Calvin cycle improves the carbon efficiency of developing green seeds". Nature 432 (7018): 779–782. doi:10.1038/nature03145. PMID 15592419. Bibcode: 2004Natur.432..779S.
- ↑ Hiatt, Howard H; Horecker, B L (13 October 1955). "D-erythrose metabolism in a strain of Alcaligenes faecalis". Journal of Bacteriology 71 (6): 649–654. doi:10.1128/jb.71.6.649-654.1956. PMID 13345750. PMC 314578. http://jb.asm.org/content/71/6/649.long. Retrieved 11 December 2014.
 |


