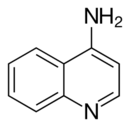
Chemistry:4-Aminoquinoline
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Quinolin-4-amine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H8N2 | |
| Molar mass | 144.177 g·mol−1 |
| Appearance | Powder to crystalline, White/Yellow/Orange |
| Melting point | 151.0 to 155.0 °C |
| Hazards | |
| Main hazards | Causes skin and serious eye irritation |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
4-Aminoquinoline is a form of aminoquinoline with the amino group at the 4-position of the quinoline. The compound has been used as a precursor for the synthesis of its derivatives.[1]
A variety of derivatives of 4-aminoquinoline are antimalarial agents useful in treating erythrocytic plasmodial infections.[2] Examples include amodiaquine, chloroquine, and hydroxychloroquine.[3] Other uses for the derivatives are: anti-asthmatic, antibacterial, anti-fungal, anti-malarial, antiviral and anti-inflammatory agents. [1]
A patent application for 4-aminoquinoline compounds was filed in 2002 and published in 2005.[4]
See also
References
- ↑ 1.0 1.1 Al-Ahmary, Khairia M.; Alenezi, Maha S.; Habeeb, Moustafa M. (2016-08-01). "Synthesis, spectroscopic and DFT theoretical studies on the hydrogen bonded charge transfer complex of 4-aminoquinoline with chloranilic acid" (in en). Journal of Molecular Liquids 220: 166–182. doi:10.1016/j.molliq.2016.04.074. ISSN 0167-7322.
- ↑ Bosak, Anita; Opsenica, Dejan M.; Šinko, Goran; Zlatar, Matija; Kovarik, Zrinka (2019-08-01). "Structural aspects of 4-aminoquinolines as reversible inhibitors of human acetylcholinesterase and butyrylcholinesterase" (in en). Chemico-Biological Interactions 308: 101–109. doi:10.1016/j.cbi.2019.05.024. ISSN 0009-2797. PMID 31100281. http://cer.ihtm.bg.ac.rs/handle/123456789/2905.
- ↑ "4-Aminoquinoline resistance of Plasmodium falciparum: insights from the study of amodiaquine uptake". Mol. Pharmacol. 50 (6): 1551–8. 1996. PMID 8967977. http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=8967977.
- ↑ DeVita, Robert; Chang, Lehua (13 January 2005). "4-Aminoquinoline Compounds". United States Patent Application Publication. https://patentimages.storage.googleapis.com/e6/5d/88/41b41326ea88f8/US20050009815A1.pdf.
External links
- "Three 4-aminoquinolines of antimalarial interest". Acta Crystallogr C 62 (Pt 2): o53–7. 2006. doi:10.1107/S0108270105041235. PMID 16456284.
- "4-Aminoquinoline 578-68-7 | TCI America". www.tcichemicals.com. Retrieved 2020-03-06.
 |