Biology:Ferritin
| Ferritin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
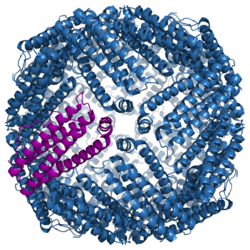 Structure of the murine ferritin complex[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Ferritin | ||||||||
| Pfam | PF00210 | ||||||||
| Pfam clan | CL0044 | ||||||||
| InterPro | IPR008331 | ||||||||
| SCOP2 | 1fha / SCOPe / SUPFAM | ||||||||
| |||||||||
| ferritin, light polypeptide | |
|---|---|
| Identifiers | |
| Symbol | FTL |
| NCBI gene | 2512 |
| HGNC | 3999 |
| OMIM | 134790 |
| RefSeq | NM_000146 |
| UniProt | P02792 |
| Other data | |
| Locus | Chr. 19 q13.3–13.4 |
| ferritin, heavy polypeptide 1 | |
|---|---|
| Identifiers | |
| Symbol | FTH1 |
| Alt. symbols | FTHL6 |
| NCBI gene | 2495 |
| HGNC | 3976 |
| OMIM | 134770 |
| RefSeq | NM_002032 |
| UniProt | P02794 |
| Other data | |
| Locus | Chr. 11 q13 |
| ferritin mitochondrial | |
|---|---|
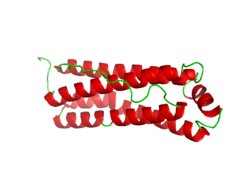 Crystallographic structure of mitochondrial ferritin.[2] | |
| Identifiers | |
| Symbol | FTMT |
| NCBI gene | 94033 |
| HGNC | 17345 |
| OMIM | 608847 |
| RefSeq | NM_177478 |
| UniProt | Q8N4E7 |
| Other data | |
| Locus | Chr. 5 q23.1 |
Ferritin is a universal intracellular protein that stores iron and releases it in a controlled fashion. The protein is produced by almost all living organisms, including archaea, bacteria, algae, higher plants, and animals. It is the primary intracellular iron-storage protein in both prokaryotes and eukaryotes, keeping iron in a soluble and non-toxic form. In humans, it acts as a buffer against iron deficiency and iron overload.[3]
Ferritin is found in most tissues as a cytosolic protein, but small amounts are secreted into the serum where it functions as an iron carrier. Plasma ferritin is also an indirect marker of the total amount of iron stored in the body; hence, serum ferritin is used as a diagnostic test for iron-deficiency anemia.[4] Aggregated ferritin transforms into a toxic form of iron called hemosiderin.[5]
Ferritin is a globular protein complex consisting of 24 protein subunits forming a hollow nanocage with multiple metal–protein interactions.[6] Ferritin that is not combined with iron is called apoferritin.[citation needed]
Gene
Ferritin genes are highly conserved between species. All vertebrate ferritin genes have three introns and four exons.[7] In human ferritin, introns are present between amino acid residues 14 and 15, 34 and 35, and 82 and 83; in addition, there are one to two hundred untranslated bases at either end of the combined exons.[8] The tyrosine residue at amino acid position 27 is thought to be associated with biomineralization.[9]
Protein structure
Ferritin is a hollow globular protein of mass 474 kDa and comprising 24 subunits. Typically it has internal and external diameters of about 8 and 12 nm, respectively.[10] The nature of these subunits varies by class of organism:
- In vertebrates, the subunits are of two types, light (L) and heavy (H), which have apparent molecular mass of 19 kDa and 21 kDa, respectively; their sequences are homologous (about 50% identical).[8]
- Amphibians have an additional ("M") type of ferritin.[11]
- Plants and bacteria have a single ferritin; it most closely resembles the vertebrate H-type.[11]
- In the gastropods of the genus Lymnaea, two types have been recovered, from somatic cells and the yolk, respectively (see below).[11]
- In the pearl oyster Pinctada fucata, an additional subunit resembling Lymnaea soma ferritin is associated with shell formation.[12]
- In the parasite Schistosoma, two types are present: one in males, the other in females.[11]
All the aforementioned ferritins are similar, in terms of their primary sequence, with the vertebrate H-type.[11] In E. coli, a 20% similarity to human H-ferritin is observed.[11] Some ferritin complexes in vertebrates are hetero-oligomers of two highly related gene products with slightly different physiological properties. The ratio of the two homologous proteins in the complex depends on the relative expression levels of the two genes.
Inside the ferritin shell, iron ions form crystallites together with phosphate and hydroxide ions. The resulting particle is similar to ferrihydrite. Each ferritin complex can store about 4500 iron (Fe3+) ions.[8][11]
A human mitochondrial ferritin, MtF, was found to express as a pro-protein.[13] When a mitochondrion takes it up, it processes it into a mature protein similar to the ferritins found in the cytoplasm, which it assembles to form functional ferritin shells. Unlike other human ferritins, it appears to have no introns in its genetic code. An X-ray diffraction study has revealed that its diameter is 1.70 angstroms (0.17 nm), it contains 182 residues, and is 67% helical. The mitochondrial ferritin's Ramachandran plot[14] shows its structure to be mainly alpha helical with a low prevalence of beta sheets.
Function
Iron storage
Ferritin is present in every cell type.[8] It serves to store iron in a non-toxic form, to deposit it in a safe form, and to transport it to areas where it is required.[15] The function and structure of the expressed ferritin protein varies in different cell types. This is controlled primarily by the amount and stability of messenger RNA (mRNA), but also by changes in how the mRNA is stored and how efficiently it is transcribed.[8] One major trigger for the production of many ferritins is the mere presence of iron;[8] an exception is the yolk ferritin of Lymnaea sp., which lacks an iron-responsive unit.[11]
Free iron is toxic to cells as it acts as a catalyst in the formation of free radicals from reactive oxygen species via the Fenton reaction.[16] Hence vertebrates have an elaborate set of protective mechanisms to bind iron in various tissue compartments[discuss]. Within cells, iron is stored in a protein complex as ferritin or the related complex hemosiderin. Apoferritin binds to free ferrous iron and stores it in the ferric state. As ferritin accumulates within cells of the reticuloendothelial system, protein aggregates are formed as hemosiderin. Iron in ferritin or hemosiderin can be extracted for release by the RE cells, although hemosiderin is less readily available. Under steady-state conditions, the level of ferritin in the blood serum correlates with total body stores of iron; thus, the serum ferritin FR5Rl is the most convenient laboratory test to estimate iron stores.[citation needed]
Because iron is an important mineral in mineralization, ferritin is employed in the shells of organisms such as molluscs to control the concentration and distribution of iron, thus sculpting shell morphology and colouration.[17][18] It also plays a role in the haemolymph of the polyplacophora, where it serves to rapidly transport iron to the mineralizing radula.[19]
Iron is released from ferritin for use by ferritin degradation, which is performed mainly by lysosomes.[20]
Ferroxidase activity
Vertebrate ferritin consists of two or three subunits which are named based on their molecular weight: L "light", H "heavy", and M "middle" subunits. The M subunit has only been reported in bullfrogs. In bacteria and archaea, ferritin consists of one subunit type.[21] H and M subunits of eukaryotic ferritin and all subunits of bacterial and archaeal ferritin are H-type and have ferroxidase activity, which is the conversion of iron from the ferrous (Fe2+) to ferric (Fe3+) forms. This limits the deleterious reaction which occurs between ferrous iron and hydrogen peroxide known as the Fenton reaction which produces the highly damaging hydroxyl radical. The ferroxidase activity occurs at a diiron binding site in the middle of each H-type subunits.[21][22] After oxidation of Fe(II), the Fe(III) product stays metastably in the ferroxidase center and is displaced by Fe(II),[22][23] a mechanism that appears to be common among ferritins of all three domains of life.[21] The light chain of ferritin has no ferroxidase activity but may be responsible for the electron transfer across the protein cage.[24]
Immune response
Ferritin concentrations increase drastically in the presence of an infection or cancer. Endotoxins are an up-regulator of the gene coding for ferritin, thus causing the concentration of ferritin to rise. By contrast, organisms such as Pseudomonas, although possessing endotoxin, cause plasma ferritin levels to drop significantly within the first 48 hours of infection. Thus, the iron stores of the infected body are denied to the infective agent, impeding its metabolism.[25]
Stress response
The concentration of ferritin has been shown to increase in response to stresses such as anoxia,[26] which implies that it is an acute phase protein.[27]
Mitochondria
Mitochondrial ferritin has many roles pertaining to molecular function. It participates in ferroxidase activity, binding, iron ion binding, oxidoreductase activity, ferric iron binding, metal ion binding as well as transition metal binding. Within the realm of biological processes it participates in oxidation-reduction, iron ion transport across membranes and cellular iron ion homeostasis.[citation needed]
Yolk
In some snails, the protein component of the egg yolk is primarily ferritin.[28] This is a different ferritin, with a different genetic sequence, from the somatic ferritin. It is produced in the midgut glands and secreted into the haemolymph, whence it is transported to the eggs.[28]
Tissue distribution
In vertebrates, ferritin is usually found within cells, although it is also present in smaller quantities in the plasma.[25]
Diagnostic uses
Serum ferritin levels are measured in medical laboratories as part of the iron studies workup for iron-deficiency anemia.[6] They are measured in nanograms per milliliter (ng/mL) or micrograms per liter (μg/L); the two units are equivalent.
The ferritin levels measured usually have a direct correlation with the total amount of iron stored in the body. However, ferritin levels may be artificially high in cases of anemia of chronic disease, where ferritin is elevated in its capacity as an inflammatory acute phase protein and not as a marker for iron overload.[citation needed]
Normal ranges
A normal ferritin blood level, referred to as the reference interval is determined by many testing laboratories. The ranges for ferritin can vary between laboratories but typical ranges would be between 40 and 300 ng/mL (=μg/L) for males, and 20–200 ng/mL (=μg/L) for females.[29]
| Adult males | 40–300 ng/mL (μg/L)[29] |
| Adult females | 20–200 ng/mL (μg/L)[29] |
| Children (6 months to 15 years) | 50–140 ng/mL (μg/L) |
| Infants (1 to 5 months) | 50–200 ng/mL (μg/L) |
| Neonates | 25–200 ng/mL (μg/L) |
Deficiency
According to a 2014 review in the New England Journal of Medicine stated that a ferritin level below 30 ng/mL indicates iron deficiency, while a level below 10 ng/mL indicates iron-deficiency anemia.[29] A 2020 World Health Organization guideline states that ferritin indicates iron deficiency below 12 ng/mL in apparently-healthy children under 5 and 15 ng/mL in apparently-healthy individuals of 5 and over.[30]
Some studies suggest that women with fatigue and ferritin below 50 ng/mL see reduced fatigue after iron supplementation.[31][32]
In the setting of anemia, low serum ferritin is the most specific lab finding for iron-deficiency anemia.[33] However it is less sensitive, since its levels are increased in the blood by infection or any type of chronic inflammation,[34] and these conditions may convert what would otherwise be a low level of ferritin from lack of iron, into a value in the normal range. For this reason, low ferritin levels carry more information than those in the normal range. A falsely low blood ferritin (equivalent to a false positive test) is very uncommon,[34] but can result from a hook effect of the measuring tools in extreme cases.[35]
Low ferritin may also indicate hypothyroidism, vitamin C deficiency or celiac disease.[citation needed]
Low serum ferritin levels are seen in some patients with restless legs syndrome, not necessarily related to anemia, but perhaps due to low iron stores short of anemia.[36][37]
Vegetarianism is not a cause of low serum ferritin levels, according to the American Dietetic Association's position in 2009: "Incidence of iron-deficiency anemia among vegetarians is similar to that of non-vegetarians. Although vegetarian adults have lower iron stores than non-vegetarians, their serum ferritin levels are usually within the normal range."[38]
Excess
If ferritin is high, there is iron in excess or else there is an acute inflammatory reaction in which ferritin is mobilized without iron excess. For example, ferritins may be high in infection without signaling body iron overload.
Ferritin is also used as a marker for iron overload disorders, such as hemochromatosis or hemosiderosis. Adult-onset Still's disease, some porphyrias, and hemophagocytic lymphohistiocytosis/macrophage activation syndrome are diseases in which the ferritin level may be abnormally raised.
As ferritin is also an acute-phase reactant, it is often elevated in the course of disease. A normal C-reactive protein can be used to exclude elevated ferritin caused by acute phase reactions. [citation needed]
Ferritin has been shown to be elevated in some cases of COVID-19 and may correlate with worse clinical outcome.[39][40] Ferritin and IL-6 are considered to be possible immunological biomarkers for severe and fatal cases of COVID-19. Ferritin and C-reactive protein may be possible screening tools for early diagnosis of systemic inflammatory response syndrome in cases of COVID-19.[41][42]
According to a study of anorexia nervosa patients, ferritin can be elevated during periods of acute malnourishment, perhaps due to iron going into storage as intravascular volume and thus the number of red blood cells falls.[43]
Another study suggests that due to the catabolic nature of anorexia nervosa, isoferritins may be released. Furthermore, ferritin has significant non-storage roles within the body, such as protection from oxidative damage. The rise of these isoferritins may contribute to an overall increase in ferritin concentration. The measurement of ferritin through immunoassay or immunoturbidimeteric methods may also be picking up these isoferritins thus not a true reflection of iron storage status.[44]
Studies reveal that a transferrin saturation (serum iron concentration ÷ total iron binding capacity) over 60 percent in men and over 50 percent in women identified the presence of an abnormality in iron metabolism (hereditary hemochromatosis, heterozygotes, and homozygotes) with approximately 95 percent accuracy. This finding helps in the early diagnosis of hereditary hemochromatosis, especially while serum ferritin still remains low. The retained iron in hereditary hemochromatosis is primarily deposited in parenchymal cells, with reticuloendothelial cell accumulation occurring very late in the disease. This is in contrast to transfusional iron overload in which iron deposition occurs first in the reticuloendothelial cells and then in parenchymal cells. This explains why ferritin levels remain relative low in hereditary hemochromatosis, while transferrin saturation is high.[45][46]
In chronic liver diseases
Hematological abnormalities often associate with chronic liver diseases. Both iron overload and iron deficient anemia have been reported in patients with liver cirrhosis.[47][48] The former is mainly due to reduced hepcidin level caused by the decreased synthetic capacity of the liver, while the latter is due to acute and chronic bleeding caused by portal hypertension. Inflammation is also present in patients with advanced chronic liver disease. As a consequence, elevated hepatic and serum ferritin levels are consistently reported in chronic liver diseases.[49][50][51]
Studies showed association between high serum ferritin levels and increased risk of short-term mortality in cirrhotic patients with acute decompensation[52] and acute-on-chronic liver failure.[53] An other study found association between high serum ferritin levels and increased risk of long-term mortality in compensated and stable decompensated cirrhotic patients.[54] The same study demonstrated that increased serum ferritin levels could predict the development of bacterial infection in stable decompensated cirrhotic patients, while in compensated cirrhotic patients the appearance of the very first acute decompensation episode showed higher incidence in patients with low serum ferritin levels. This latter finding was explaind by the association between chronic bleeding and increased portal pressure.[54]
Applications
Ferritin is used in materials science as a precursor in making iron nanoparticles for carbon nanotube growth by chemical vapor deposition.
Cavities formed by ferritin and mini-ferritins (Dps) proteins have been successfully used as the reaction chamber for the fabrication of metal nanoparticles (NPs).[55][56][57][58] Protein shells served as a template to restrain particle growth and as a coating to prevent coagulation/aggregation between NPs. Using various sizes of protein shells, various sizes of NPs can be easily synthesized for chemical, physical and bio-medical applications.[6][59]
Experimental COVID-19 vaccines have been produced that display the spike protein's receptor binding domain on the surface of ferritin nanoparticles.[60]
Notes
The primary sequence of human ferritin is MTTASTSQVR QNYHQDSEAA INRQINLELY ASYVYLSMSY YFDRDDVALK NFAKYFLHQS HEEREHAEKL MKLQNQRGGR IFLQDIKKPD CDDWESGLNA MECALHLEKN VNQSLLEFPS PISPSPSCWH HYTTNRPQPQ HHLLRPRRRK RPHSIPTPIL IFRSP.[61]
See also
- Bacterioferritin
- DNA-binding protein from starved cells
- Ferritin light chain
- Transferrin
References
- ↑ PDB: 1lb3; "Structural description of the active sites of mouse L-chain ferritin at 1.2 A resolution". Journal of Biological Inorganic Chemistry 8 (1–2): 105–11. January 2003. doi:10.1007/s00775-002-0389-4. PMID 12459904.
- ↑ PDB: 1r03; "Crystal structure and biochemical properties of the human mitochondrial ferritin and its mutant Ser144Ala". Journal of Molecular Biology 340 (2): 277–93. July 2004. doi:10.1016/j.jmb.2004.04.036. PMID 15201052.
- ↑ "Iron Use and Storage in the Body: Ferritin and Molecular Representations". Department of Chemistry, Washington University in St. Louis. http://www.chemistry.wustl.edu/~edudev/LabTutorials/Ferritin/Ferritin.html.
- ↑ "Serum ferritin: Past, present and future". Biochimica et Biophysica Acta (BBA) - General Subjects 1800 (8): 760–9. August 2010. doi:10.1016/j.bbagen.2010.03.011. PMID 20304033.
- ↑ "Intracellular iron transport and storage: from molecular mechanisms to health implications". Antioxidants & Redox Signaling 10 (6): 997–1030. June 2008. doi:10.1089/ars.2007.1893. PMID 18327971.
- ↑ 6.0 6.1 6.2 "Ferritin protein nanocages-the story". Nanotechnology Perceptions 8 (1): 7–16. 2012. doi:10.4024/N03TH12A.ntp.08.01. PMID 24198751.
- ↑ "Regulation of ferritin genes and protein". Blood 99 (10): 3505–16. May 2002. doi:10.1182/blood.V99.10.3505. PMID 11986201.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 "Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms". Annual Review of Biochemistry 56 (1): 289–315. 1987. doi:10.1146/annurev.bi.56.070187.001445. PMID 3304136.
- ↑ "Two ferritin subunits from disk abalone (Haliotis discus discus): cloning, characterization and expression analysis". Fish & Shellfish Immunology 23 (3): 624–35. September 2007. doi:10.1016/j.fsi.2007.01.013. PMID 17442591.
- ↑ "Ferritin Structure and Its Biomedical Implications". Universidad de Granada. http://www.ugr.es/~josema/Structure.html.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 "Structure, function, and evolution of ferritins". Journal of Inorganic Biochemistry 47 (3–4): 161–74. 1992. doi:10.1016/0162-0134(92)84062-R. PMID 1431878.
- ↑ "A novel ferritin subunit involved in shell formation from the pearl oyster (Pinctada fucata)". Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology 135 (1): 43–54. May 2003. doi:10.1016/S1096-4959(03)00050-2. PMID 12781972.
- ↑ "A human mitochondrial ferritin encoded by an intronless gene". The Journal of Biological Chemistry 276 (27): 24437–40. July 2001. doi:10.1074/jbc.C100141200. PMID 11323407.
- ↑ "Structure validation by Calpha geometry: phi,psi and Cbeta deviation". Proteins 50 (3): 437–50. February 2003. doi:10.1002/prot.10286. PMID 12557186. http://www.rcsb.org/pdb/images/1R03_ram_m_500.pdf. "MolProbity Ramachandran analysis".
- ↑ "Ferreting out the secrets of plant ferritin - A review". Journal of Plant Nutrition 5 (4–7): 369–394. 1982. doi:10.1080/01904168209362966.
- ↑ "Ferritin and the response to oxidative stress". The Biochemical Journal 357 (Pt 1): 241–7. July 2001. doi:10.1042/0264-6021:3570241. PMID 11415455.
- ↑ "Dynamic expression of ancient and novel molluscan shell genes during ecological transitions". BMC Evolutionary Biology 7: 160. 2007. doi:10.1186/1471-2148-7-160. PMID 17845714.
- ↑ "Shematrin: a family of glycine-rich structural proteins in the shell of the pearl oyster Pinctada fucata". Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology 144 (2): 254–62. June 2006. doi:10.1016/j.cbpb.2006.03.004. PMID 16626988.
- ↑ "Properties and role of ferritin in the hemolymph of the chiton Clavarizona hirtosa". Biochimica et Biophysica Acta (BBA) - General Subjects 884 (3): 387–394. 1986. doi:10.1016/0304-4165(86)90188-1.
- ↑ "Lysosomal proteolysis is the primary degradation pathway for cytosolic ferritin and cytosolic ferritin degradation is necessary for iron exit". Antioxidants & Redox Signaling 13 (7): 999–1009. October 2010. doi:10.1089/ars.2010.3129. PMID 20406137.
- ↑ 21.0 21.1 21.2 "Unity in the biochemistry of the iron-storage proteins ferritin and bacterioferritin". Chemical Reviews 115 (1): 295–326. January 2015. doi:10.1021/cr5004908. PMID 25418839.
- ↑ 22.0 22.1 "The catalytic center of ferritin regulates iron storage via Fe(II)-Fe(III) displacement". Nature Chemical Biology 8 (11): 941–8. November 2012. doi:10.1038/nchembio.1071. PMID 23001032.
- ↑ "A unified model for ferritin iron loading by the catalytic center: implications for controlling "free iron" during oxidative stress". ChemBioChem 14 (4): 415–9. March 2013. doi:10.1002/cbic.201200783. PMID 23404831.
- ↑ "Ferritin light-chain subunits: key elements for the electron transfer across the protein cage". Chemical Communications 50 (97): 15358–61. December 2014. doi:10.1039/c4cc07996e. PMID 25348725.
- ↑ 25.0 25.1 "The response of ferritin to LPS and acute phase of Pseudomonas infection". Journal of Endotoxin Research 11 (5): 267–80. 2005. doi:10.1179/096805105X58698. PMID 16262999.
- ↑ "Accumulation and translation of ferritin heavy chain transcripts following anoxia exposure in a marine invertebrate". The Journal of Experimental Biology 207 (Pt 8): 1353–60. March 2004. doi:10.1242/jeb.00872. PMID 15010486.
- ↑ "Evolution of the acute phase response: iron release by echinoderm (Asterias forbesi) coelomocytes, and cloning of an echinoderm ferritin molecule". Developmental and Comparative Immunology 26 (1): 11–26. January 2002. doi:10.1016/S0145-305X(01)00051-9. PMID 11687259.
- ↑ 28.0 28.1 "On the origin of the yolk protein ferritin in snails". Roux's Archives of Developmental Biology 197 (7): 377–382. 1988. doi:10.1007/BF00398988. PMID 28305744.
- ↑ 29.0 29.1 29.2 29.3 Camaschella, Clara (2015-05-07). Longo, Dan L.. ed. "Iron-Deficiency Anemia" (in en). New England Journal of Medicine 372 (19): 1832–1843. doi:10.1056/NEJMra1401038. ISSN 0028-4793. PMID 25946282. http://www.nejm.org/doi/10.1056/NEJMra1401038.
- ↑ World Health Organization (2020). WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations.. Geneva, Switzerland. ISBN 978-92-4-000012-4. OCLC 1265083396. https://www.worldcat.org/oclc/1265083396.
- ↑ "Iron supplementation for unexplained fatigue in non-anaemic women: double blind randomised placebo controlled trial.". BMJ (Clinical Research Ed.) 326 (7399): 1124–0. May 2003. doi:10.1136/bmj.326.7399.1124. PMID 12763985.
- ↑ "Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial.". CMAJ: Canadian Medical Association Journal 184 (11): 1247–1254. August 2012. doi:10.1503/cmaj.110950. PMID 22777991.
- ↑ "Diagnosis of iron-deficiency anemia in the elderly". The American Journal of Medicine 88 (3): 205–9. March 1990. doi:10.1016/0002-9343(90)90143-2. PMID 2178409.
- ↑ 34.0 34.1 "Interpretation of biochemical tests for iron deficiency: diagnostic difficulties related to limitations of individual tests". Australian Prescriber 20: 74–6. 1997. doi:10.18773/austprescr.1997.063. http://www.australianprescriber.com/magazine/20/3/74/6.
- ↑ The Science of Laboratory Diagnosis. ISIS Medical Media. 1999. pp. 341. ISBN 978-1-899066-62-9. https://books.google.com/books?id=9VBFVkMX3N0C&pg=PA236.
- ↑ "Low body stores of iron and restless legs syndrome: a correctable cause of insomnia in adolescents and teenagers". Sleep Medicine 3 (2): 127–32. March 2002. doi:10.1016/S1389-9457(01)00160-5. PMID 14592231.
- ↑ "CSF iron, ferritin and transferrin levels in restless legs syndrome". Journal of Sleep Research 14 (1): 43–7. March 2005. doi:10.1111/j.1365-2869.2004.00403.x. PMID 15743333.
- ↑ "Position of the American Dietetic Association: vegetarian diets". Journal of the American Dietetic Association 109 (7): 1266–82. July 2009. doi:10.1016/j.jada.2009.05.027. PMID 19562864. https://digitalcommons.andrews.edu/pubs/1954.
- ↑ "Clinical and immunological features of severe and moderate coronavirus disease 2019". The Journal of Clinical Investigation 130 (5): 2620–2629. May 2020. doi:10.1172/JCI137244. PMID 32217835.
- ↑ "Correlation between Micronutrient plasma concentration and disease severity in COVID-19 patients". Alexandria Journal of Medicine 57 (1): 21–27. 2021. doi:10.1080/20905068.2020.1870788. ISSN 2090-5068.
- ↑ "Biomarkers of cytokine storm as red flags for severe and fatal COVID-19 cases: A living systematic review and meta-analysis". PLOS ONE 16 (6): e0253894. 29 June 2021. doi:10.1371/journal.pone.0253894. PMID 34185801. Bibcode: 2021PLoSO..1653894M.
- ↑ "What is a cytokine storm?". Annual Reviews. 10 April 2020. https://knowablemagazine.org/article/health-disease/2020/what-cytokine-storm.
- ↑ "Iron status and haematological changes in adolescent female inpatients with anorexia nervosa". Journal of Paediatrics and Child Health 40 (8): 430–2. August 2004. doi:10.1111/j.1440-1754.2004.00432.x. PMID 15265182.
- ↑ "Unexpected increased ferritin concentration in patients with anorexia nervosa". Annals of Clinical Biochemistry 50 (Pt 5): 504–6. September 2013. doi:10.1177/0004563213490289. PMID 23897102.
- ↑ "Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases". Hepatology (Baltimore, Md.) 54 (1): 328–43. July 2011. doi:10.1002/hep.24330. PMID 21452290.
- ↑ "Hemochromatosis". guidelinecentral.com. http://eguideline.guidelinecentral.com/i/98549-aasld-hemochromatosis/2.
- ↑ "End-stage liver disease without hemochromatosis associated with elevated hepatic iron index" (in English). Journal of Hepatology 29 (2): 257–62. August 1998. doi:10.1016/S0168-8278(98)80011-1. PMID 9722207.
- ↑ "Iron deficiency anemia in chronic liver disease: etiopathogenesis, diagnosis and treatment". Annals of Gastroenterology 30 (4): 405–413. 2017. doi:10.20524/aog.2017.0152. PMID 28655976.
- ↑ "Liver iron is a surrogate marker of severe fibrosis in chronic hepatitis C". Journal of Hepatology 46 (4): 587–95. April 2007. doi:10.1016/j.jhep.2006.09.021. PMID 17156889.
- ↑ "Iron overload in viral and alcoholic liver disease". Journal of Hepatology 28 (Suppl 1): 19–20. 1998. doi:10.1016/S0168-8278(98)80370-X. PMID 9575444.
- ↑ "Hepatic iron overload in alcoholic end-stage liver disease is associated with iron deposition in other organs in the absence of HFE-1 hemochromatosis". Liver International 25 (3): 513–7. June 2005. doi:10.1111/j.1478-3231.2005.01004.x. PMID 15910487.
- ↑ "Serum ferritin predicts early mortality in patients with decompensated cirrhosis". Journal of Hepatology 61 (1): 43–50. July 2014. doi:10.1016/j.jhep.2014.03.027. PMID 24681346.
- ↑ "Dysregulated iron homeostasis is strongly associated with multiorgan failure and early mortality in acute-on-chronic liver failure". Hepatology 61 (4): 1306–20. April 2015. doi:10.1002/hep.27636. PMID 25475192.
- ↑ 54.0 54.1 "Abnormal ferritin levels predict development of poor outcomes in cirrhotic outpatients: a cohort study". BMC Gastroenterology 21 (1): 94. March 2021. doi:10.1186/s12876-021-01669-w. PMID 33653274.
- ↑ "Silver Ion Incorporation and Nanoparticle Formation inside the Cavity ofPyrococcus furiosusFerritin: Structural and Size-Distribution Analyses". Journal of the American Chemical Society 132 (10): 3621–7. March 2010. doi:10.1021/ja910918b. PMID 20170158. https://www.openaccessrepository.it/record/21528.
- ↑ "Targeting of Cancer Cells with Ferrimagnetic Ferritin Cage Nanoparticles". Journal of the American Chemical Society 128 (51): 16626–33. December 2006. doi:10.1021/ja0655690. PMID 17177411.
- ↑ "Polymer-Mediated Synthesis of Ferritin-Encapsulated Inorganic Nanoparticles". Small 3 (9): 1477–81. September 2007. doi:10.1002/smll.200700199. PMID 17768776.
- ↑ "Size-Selective Olefin Hydrogenation by a Pd Nanocluster Provided in an Apo-Ferritin Cage". Angewandte Chemie 43 (19): 2527–30. May 2004. doi:10.1002/anie.200353436. PMID 15127443.
- ↑ Stanford's Single-Dose Nanoparticle Vaccine for COVID-19. On: SciTechDaily. January 10, 2021. Source: Stanford University
- ↑ "Ferritin nanoparticle-based SARS-CoV-2 RBD vaccine induces a persistent antibody response and long-term memory in mice". Cellular & Molecular Immunology 18 (3): 749–751. March 2021. doi:10.1038/s41423-021-00643-6. PMID 33580169.
- ↑ "Ferritin - Homo sapiens (Human)". https://www.uniprot.org/uniprot/Q8TD27.
External links
- Ferritins at the US National Library of Medicine Medical Subject Headings (MeSH)
- Ferritin at Lab Tests Online
- Overview of all the structural information available in the PDB for UniProt: P02792 (Ferritin light chain) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: P02794 (Ferritin heavy chain) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: Q8N4E7 (Ferritin, mitochondrial) at the PDBe-KB.


