Chemistry:Cymene
From HandWiki
Cymene describes organic compounds with the formula CH
3C
6H
4CH(CH
3)
2. Three isomers exist: 1,2- 1,3-, and 1,4-. All are colorless liquids, immiscible in water, with similar boiling points. They are classified are aromatic hydrocarbons. The bearing two substituents: an isopropyl (CH(CH
3)
2) group and a methyl group.[1]
| Cymenes | |||
| Name | o-Cymene | m-Cymene | p-Cymene |
|---|---|---|---|
| Structural formula | 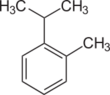 |
110px | 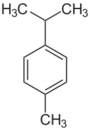
|
| CAS number | 527-84-4 | 535-77-3 | 99-87-6 |
| melting point | -71.54 | -63.75 | -67.94 |
| boiling point (°C) | 178.15 | 175.05 | 177.10 |
Production and reactions
m- and p-Cymene are prepared by alkylation of toluene with propylene:
- CH
3C
6H
5 + 2 CH
3CH=CH
2 → CH
3C
6H
4CH(CH
3)
2
These alkylations are catalyzed by various Lewis acids, such as aluminium trichloride.
m- and p-Cymene are mainly of interest as precursors to the respective cresols, which exploits the Hock rearrangements.[1]
References
- ↑ 1.0 1.1 Schmidt, Roland; Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd et al. (2014). "Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. pp. 1–74. doi:10.1002/14356007.a13_227.pub3. ISBN 9783527306732.
 |

