Chemistry:M-Cymene
From HandWiki
Short description: Organic compound
| |
| Names | |
|---|---|
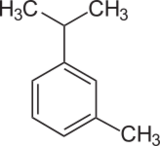
| Preferred IUPAC name
1-Methyl-3-(propan-2-yl)benzene | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H14 | |
| Molar mass | 134.22 |
| Appearance | colorless liquid |
| Density | 0.86 g/cm3 |
| Melting point | −63.8 °C (−82.8 °F; 209.3 K) |
| Boiling point | 175 °C (347 °F; 448 K) |
| 42.5 mg/L | |
| Hazards | |
| Main hazards | Flammable |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H226 | |
| P210, P233, P240, P241, P242, P243, P280, P303+361+353, P370+378, P403+235, P501 | |
| Flash point | 47.8 °C (118.0 °F; 320.9 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
m-Cymene is an organic compound classified as an aromatic hydrocarbon. Its structure consists of a benzene ring meta-substituted with a methyl group and an isopropyl group. It is a flammable colorless liquid which is nearly insoluble in water but soluble in organic solvents.
Isomers and production
In addition to m-cymene, there are two other geometric isomers called o-cymene, in which the alkyl groups are ortho-substituted, and p-cymene, in which they are para-substituted. p-Cymene is the most common and only natural isomer. The three isomers form the group of cymenes.
Cymenes can be produced by alkylation of toluene with propylene.[1][2]
References
- ↑ Vora, Bipin V.; Kocal, Joseph A.; Barger, Paul T.; Schmidt, Robert J.; Johnson, James A. (2003). "Kirk-Othmer Encyclopedia of Chemical Technology". Kirk‐Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0112112508011313.a01.pub2. ISBN 0471238961.
- ↑ Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter et al. (2002). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_227.
 |
