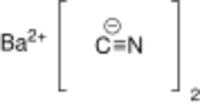
Chemistry:Barium cyanide
| |
| Names | |
|---|---|
| IUPAC name
Barium dicyanide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Ba(CN)2 | |
| Molar mass | 189.362 g/mol |
| Appearance | white crystalline powder |
| Melting point | 600 °C (1,112 °F; 873 K) |
| 18 g/100 mL (14 °C) | |
| Solubility | Soluble in ethanol |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | DANGER |
| H300, H310, H330, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+316Script error: No such module "Preview warning".Category:GHS errors, P302+352, P304+340, P316Script error: No such module "Preview warning".Category:GHS errors, P320, P321, P330, P361+364Script error: No such module "Preview warning".Category:GHS errors, P391, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Barium cyanide is a chemical compound with the formula Ba(CN)2. It is synthesized by the reaction of hydrogen cyanide and barium hydroxide in water or petroleum ether.[1] It is a white crystalline salt.
Uses
Barium cyanide is used in electroplating and other metallurgical processes.
Reactions
Barium cyanide reacts with water and carbon dioxide in air slowly, producing highly toxic hydrogen cyanide gas.[2]
When barium cyanide is heated to 300°C with steam present, the nitrogen evolves to ammonia, leaving barium formate.[citation needed]
- Ba(CN)2 + 4 H2O = Ba(HCOO)2 + 2 NH3
Aqueous solutions of barium cyanide dissolve insoluble cyanides of some of the heavy metals forming crystalline double salts. For example, BaHg(CN)4.3H2O in needles, 2Ba(CN)2.3Hg(CN)2.23H2O in transparent octahedra, and Ba(CN)2.Hg(CN)2.HgI2.6H2O.[3]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Smith, R P; Gosselin, R E (1976). "Current Concepts about the Treatment of Selected Poisonings: Nitrite, Cyanide, Sulfide, Barium, and Quinidine". Annual Review of Pharmacology and Toxicology 16: 189–99. doi:10.1146/annurev.pa.16.040176.001201. PMID 779614.
- ↑ "Barium Cyanide, Ba(CN)2". Atomistry. http://barium.atomistry.com/barium_cyanide.html. Retrieved 2012-11-01.
 |


