Biology:Evidence for speciation by reinforcement
| Part of a series on |
| Evolutionary biology |
|---|
 |
Reinforcement is a process within speciation where natural selection increases the reproductive isolation between two populations of species by reducing the production of hybrids.[1][2] Evidence for speciation by reinforcement has been gathered since the 1990s, and along with data from comparative studies and laboratory experiments, has overcome many of the objections to the theory.[3]:354[4][5] Differences in behavior or biology that inhibit formation of hybrid zygotes are termed prezygotic isolation. Reinforcement can be shown to be occurring (or to have occurred in the past) by measuring the strength of prezygotic isolation in a sympatric population in comparison to an allopatric population of the same species.[3]:357 Comparative studies of this allow for determining large-scale patterns in nature across various taxa.[3]:362 Mating patterns in hybrid zones can also be used to detect reinforcement.[6] Reproductive character displacement is seen as a result of reinforcement,[7] so many of the cases in nature express this pattern in sympatry. Reinforcement's prevalence is unknown,[4] but the patterns of reproductive character displacement are found across numerous taxa (vertebrates, invertebrates, plants, and fungi), and is considered to be a common occurrence in nature.[6] Studies of reinforcement in nature often prove difficult, as alternative explanations for the detected patterns can be asserted.[3]:358 Nevertheless, empirical evidence exists for reinforcement occurring across various taxa[7] and its role in precipitating speciation is conclusive.[8]
Evidence from nature
Amphibians

The two frog species Litoria ewingi and L. verreauxii live in southern Australia with their two ranges overlapping. The species have very similar calls in allopatry, but express clinal variation in sympatry, with notable distinctness in calls that generate female preference discrimination.[8] The zone of overlap sometimes forms hybrids and is thought to originate by secondary contact of once fully allopatric populations.[8]
Allopatric populations of Gastrophryne olivacea and G. carolinensis have recently come into secondary contact due to forest clearing.[9] The calls that the males make to attract females differ significantly in frequency and duration in the area where the two species overlap, despite them having similar calls where they do not.[3]:359 Further, the hybrids that form in sympatry have calls that are intermediate between the two.[9] Similar patterns of reproductive character displacement involving acoustic displays have been found in Hyla cinerea and H. gratiosa, with greater female preference for conspecific males in areas of sympatry.[10]
Three species of true frogs (Lithobates sphenocephalus, L. berlandieri, and L. blairi) are temporally isolated in that their breeding seasons are spaced out in areas where they live in sympatry, but not where they live in allopatry.[11] Selection against interspecific mating due to low hybrid fitness and low hybrid fertility has reinforced the observed character displacement of breeding times.[11]
The rainforests of northeast Queensland, Australia were separated into north and south refugia by climate fluctuations of the Pliocene and Pleistocene.[12] About 6500 years ago, the rainforests reconnected, bringing the diverged, incipient populations of Litoria genimaculata into secondary contact. The species contact zones exhibit, "strong postzygotic selection against hybrids" and enhanced isolation from differences in mating call.[13]
An alternative to detecting reproductive character displacement in populations that overlap in sympatry is measuring rates of hybridization in contact zones.[9] The frog species Anaxyrus americanus and Anaxyrus woodhousii have shown a decrease in hybridization from 9%–0% over approximately 30 years.[14][9] A similar pattern was detected in the sympatric spadefoot toads Spea multiplicata and S. bombifrons have hybridized with decreasing frequency over a 27-year period (about 13 generations).[15]
Birds

The Ficedula flycatchers exhibit a pattern that suggests premating isolation is being reinforced by sexual selection.[16] The pied flycatcher (Ficedula hypoleuca) has brown females, brown males, and black-and-white males. The related collard flycatcher (Ficedula albicollis) has brown females and only black-and-white males. The two species exist in separate populations that overlap in a zone of sympatry.[16] In the range of overlap, only brown males of F. hypoleuca exist and are thought to have evolved the brown plumage to prevent hybridization,[17] though there is also evidence indicating that such character displacement is explained by heterospecific competition for territory rather than reinforcement.[18] Mating choice tests of the species find that females of both species choose conspecific males in sympatry, but heterospecific males in allopatry[16] (see conspecific song preference). The patterns could suggest mimicry, driven by interspecific competition;[3]:361 however, song divergence has been detected that shows a similar pattern to the mating preferences.[19]
Geospiza fuliginosa and G. difficilis males on the Galápagos Islands show a noted preference for conspecific females where they meet in sympatry, but not in allopatry.[20] Other birds such as the dark and light subspecies of the western grebe show enhanced prezygotic isolation.[21] It has been argued that reinforcement is extremely common in birds and has been documented in a wide range of bird species.[22]
Crustaceans
Reproductive character displacement in body size was detected in sympatric populations of Orconectes rusticus and O. sanbornii.[23]
Echinoderms
An example of gametic isolation involves the allopatric sea urchins (Arbacia) have minimal bindin differences (bindin is a protein involved in the process of sea urchin fertilization, used for species-specific recognition of the egg by the sperm) and have insufficient barriers to fertilization.[3]:243 Comparison with the sympatric species Echinometra and Strongylocentrotus of the Indo-Pacific finds that they have significant differences in bindin proteins for fertilization and marked fertilization barriers.[24]

Laboratory matings of closely related sea urchin species Echinometra oblonga and E. sp. C (the species is unnamed, dubbed C) produce fertile and viable hybrids, but are unable to fertilize eggs of the parent species due to divergence of the alleles that code for bindin proteins: an example of post-zygotic isolation.[3]:343–344 Populations in sympatry manifest this difference in bindin protein versus those in allopatry.[3]:343–344 Selection actively acts against the formation of hybrids in both nature (as no documented cases of hybrids have been found) and in the laboratory.[25] Here, the evolution of female egg receptors is thought to pressure bindin evolution in a selective runaway process.[25] This example of reproductive character displacement is highly suggestive of being the result of—and has been cited as strong evidence for—reinforcement.[25][3]:343–344
Fish
In British Columbia, benthic and limnetic morphs of Gasterosteus aculeatus exist together in sympatry in some lakes, while containing only one morph in other lakes.[26] Female benthic morphs in sympatric populations actively discriminate against limnetic males, resulting in low rates of crossing (some gene flow has occurred between the morphs) and low fitness hybrids.[3]:360 Both selection against hybrids and reproductive character displacement in egg fertilization is observed in Etheostoma lepidum and E. spectabile.[27]
Fungi
A strong case of reinforcement occurring in fungi comes from studies of Neurospora.[28] In crosses between different species in the genera, sympatric pairs show low reproductive success, significantly lower than allopatric pairs.[28] This pattern is observed across small and large geographic scales, with distance correlating with reproductive success.[28] Further evidence of reinforcement in the species was the low fitness detected in the hybrids create from crosses, and that no hybrids have been found in nature, despite close proximity.[28]
Insects
Ethological isolation has been observed between some mosquito species in the Southeast Asian Aedes albopictus group, suggesting—from laboratory experiments of mating trials—that selection against hybrids is occurring, in the presence of reproductive character displacement.[29]
Female mate discrimination is increased with intermediate migration rates between allopatric populations of Timema cristinae (genus Timema) compared to high rates of migration (where gene flow impedes selection) or low rates (where selection is not strong enough).[30][31]
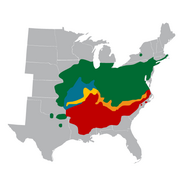
Where the ranges of the cicada species Magicicada tredecim and M. neotredecim overlap (where they are sympatric), the pitch of M. neotredecim male calling songs is roughly 1.7 kHz compared to 1.1 kHz for those of M. tredecim, with corresponding female song pitch preference differences.[32] In allopatric M. neotredecim populations, the mating call pitch is 1.3–1.5 kHz.[32] The biogeography of the cicadas suggests that M. neotredecim originated after the retreat of the last glacial advance in North America.[32]
The song differences of Laupala crickets on the Hawaiian Islands appear to exhibit patterns consistent with character displacement in sympatric populations.[33] A similar pattern exists with Allonemobius fasciatus and A. socius, species of ground crickets in eastern North America.[34]
Males in sympatric populations of the damselflies Calopteryx maculata and C. aequabilis are able to discriminate between females of different species better than those in allopatric populations; with females of C. aequabilis in sympatric populations exhibiting lighter wing colors compared to allopatric females—an illustration of reproductive character displacement.[35][36]
Fifteen species of sympatrically distributed Agrodiaetus butterflies with pronounced differences in wing color pattern likely arose as a result of speciation by reinforcement.[37] Phylogenetic patterns indicate the differences arose in allopatry and were reinforced when the distributions came into secondary contact.[38]
Drosophila
Drosophila is one of the most studied species in speciation research.[39] Dobzhansky and Koller were the first to study isolation between Drosophila species.[3]:358 Since then, other studies of natural populations such as the D. paulistorum races exhibiting stronger isolation in sympatry versus allopatry,[40] or the enhanced isolation found in sympatric populations of D. mojavensis and D. arizonae in southwest America.[41] Rare, sterile hybrids form between D. pseudoobscura and D. persimilis, with sympatric D. pseudoobscura females discriminating against D. persimilis males; more so than allopatric populations.[42] Other Drosophila research on reinforcement has been from laboratory experiments and is discussed below. On the east coast of Australia, D. serrata shares a zone of sympatric overlap with the closely relates species D. birchii.[43] The species exhibits reproductive character displacement, with sexual selection operating on the hydrocarbons of the flies cuticle.[44] Reinforcement appears to be driving their speciation in nature, supported by simulated experimental laboratory populations.[45][46]
Mammals
The deer mice Peromyscus leucopus and P. gossypinus exhibit reproductive character displacement in mating preferences, with heterospecific matings taking place between the species.[47]
Molluscs

Partula suturalis is polymorphic for shell chirality in that it has two forms: sinistral (left-handed) and dextral (right-handed) shells, unlike other monomorphic species on the island of Mo'orea which have only one form (with the exception of P. otaheitana).[48] This polymorphic trait has a direct effect on mate choice and mating behavior; as shown in laboratory mating tests that opposite-coil pairs mate much less often.[48] In areas where P. suturalis lives sympatrically with other sinistral and dextral Partula species, the opposite P. suturalis morph is typically present.[9] Butlin succinctly describes one example of this unique pattern:
P. suturalis is sympatric with the dextral P. aurantia and sinistral P. olympia, whose ranges abut but do not overlap; P. suturalis is sinestral in the range of P. aurantia and dextral in the range of P. olympia and does not normally hybridize with either species. However, where their ranges meet there is a sharp transition in the coil of P. suturalis and in this transition zone it hybridizes with both P. aurantia and P. olympia.[9]
The reversal in chirality to sinistrality must have evolved as an isolating mechanism,[49] with patterns of reproductive character displacement suggesting speciation by reinforcement.[48]
Satsuma largillierti lives on the western half of Okinawa Island while Satsuma eucosmia lives on the eastern half. Both populations overlap in sympatry along the middle of the island, where the penis length of the species differs significantly in sympatry (a case of reproductive character displacement[50]), but not in allopatry.[51] A similar pattern in snails is found with Lymnaea peregra and L. ovata in the Switzerland lake Seealpsee; with mating signal acting as the sympatrically displaced trait.[52]
The abalone genus Haliotis has 19 species that occur in sympatry and one that occurs in allopatry. Of the sympatric species, they all contain sperm lysin that drives gamete isolation, but the allopatric species does not.[53][3]:343 A similar pattern of sperm lysin differentiation is found in the mussel species Mytilus galloprovincialis and M. trossulus and has likely occurred within the last 200 years due to human-mediated distribution by ships.[3]:343
Plants
Plants are thought to provide suitable conditions for reinforcement to occur.[5] This is due to a number of factors such as the unpredictability of pollination, pollen vectors, hybridization, hybrid zones, among others.[5] The study of plants experiencing speciation by reinforcement has largely been overlooked by researchers;[3]:364 however, there is evidence of its occurrence in them.[54]
In the Texas wildflower Phlox drummondii, cis-regulatory mutations of genes that code for anthocyanin pigmentation have caused genetic divergence of two populations.[55] Hybrids (between P. drummondii and P. cuspidata) with maladaptive, intermediate characteristics are under-pollinated; increasing reproductive isolation through reinforcement.[55] The maintenance of the ancestral flower color in the allopatric population is favored weakly by selection, where the derived color in the sympatric population is being driven by strong selection.[56] Similarly, in P. pilosa and P. glaberrima, character displacement of petal color has been driven by selection, aided by pollen discrimination.[57] Displacement in flower size has also been observed in the nightshade species Solanum grayi and S. lymholtzianum in sympatry as well as S. rostratum and S. citrullifolium.[58]
The bishop pine is divided into two populations distinguished by monoterpene, stomata, and alloenzyme differences; flowering time; and needle color: blue foliage in the northern population and green foliage in the southern populations in California.[59] A small region exists where the species meet in a cline—sustained by selection due to a flowering time divergence, thought to represent reinforcement taking place.[9]
Similar patterns of both character displacement in sympatric populations of species have been documented in:[9][3]:361
- Agrostis tenuis[60]
- Anthoxanthum odoratum[60]
- Gilia[61]
- Costus plants: Costus allenii, C. laevis, and C. guanaiensis;[62][63] C. pulverulentus and C. scaber[64]
- A unique case of post-zygotic instead of prezygotic isolation has been observed in both Gossypium and Gilia, suggesting that in plants, post-zygotic isolation's role in reinforcement may play a larger role.[3]:361
- Sympatric populations of Juncus effusus (common rush) exhibits genetic differentiation of plants that flower at different times preventing hybridization.[65] Allochrony may play a role.[66]
Comparative studies
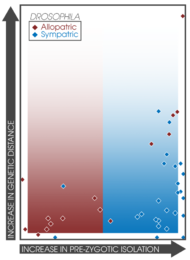
Assortive mating is expected to increase among sympatric populations experiencing reinforcement.[8] This fact allows for the direct comparison of the strength of prezygotic isolation in sympatry and allopatry between different experiments and studies.[3]:362 Jerry Coyne and H. Allen Orr surveyed 171 species pairs, collecting data on their geographic mode, genetic distance, and strength of both prezygotic and post-zygotic isolation; finding that prezygotic isolation was significantly stronger in sympatric pairs, correlating with the ages of the species.[3]:362 Additionally, the strength of post-zygotic isolation was not different between sympatric and allopatric pairs.[8]
This finding lends support the predictions of speciation by reinforcement and correlates well with another later study by Daniel J. Howard.[3]:363 In his study, 48 studies with observed reproductive character displacement (including plants, insects, crustaceans, molluscs, fish, amphibians, reptiles, birds, and mammals) were analyzed.[6] The cases met several criteria such as the trait in question serving as a reproductive barrier and if there existed clear patterns of sympatry versus allopatry.[6] Out of the 48 candidates, 69 percent (33 cases) found enhanced isolation in sympatry, suggesting that the pattern predicted by reinforcement is common in nature.[6] In addition to Howard's comparative study, he guarded against the potential for positive-result publication bias by surveying 37 studies of hybrid zones. A prediction of reinforcement is that assortive mating should be common in hybrid zones; a prediction that was confirmed in 19 of the 37 cases.[6]
A survey of the rates of speciation in fish and their associated hybrid zones found similar patterns in sympatry, supporting the occurrence of reinforcement.[68] One study in the plants Glycine and Silene; however, did not find enhanced isolation.[69]
Laboratory experiments
Laboratory studies that explicitly test for reinforcement are limited.[3]:357 In general, two types of experiments have been conducted: using artificial selection to mimic natural selection that eliminates the hybrids (often called "destroy-the-hybrids"), and using disruptive selection to select for a trait (regardless of its function in sexual reproduction).[3]:355–357 Many experiments using the destroy-the-hybrids technique are generally cited as supportive of reinforcement; however, some researchers such as Coyne and Orr and William R. Rice and Ellen E. Hostert contend that they do not truly model reinforcement, as gene flow is completely restricted between two populations.[70][3]:356 The table below summarizes some of the laboratory experiments that are often cited as testing reinforcement in some form.
| Species | Experimental design | Result | Year |
|---|---|---|---|
| D. paulistorum | Destroyed hybrids | Pre-zygotic isolation | 1976[71] |
| D.pseudoobscura &
D. persimilis |
Destroyed hybrids | Pre-zygotic isolation; reproductive character displacement | 1950[72] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation; reproductive character displacement | 1974[73] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation; reproductive character displacement | 1956[74] |
| D. melanogaster | Destroyed hybrids | No pre-zygotic isolation detected | 1970[75] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation | 1953[76] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation | 1974[77] |
| D. melanogaster | Allopatric populations in secondary contact | N/A | 1982[78] |
| D. melanogaster | N/A | 1991[79] | |
| D. melanogaster | No pre-zygotic isolation detected | 1966[80][81] | |
| D. melanogaster | Allowed gene flow between populations | No pre-zygotic isolation detected | 1969[82] |
| D. melanogaster | N/A | No pre-zygotic isolation detected | 1984[83] |
| D. melanogaster | Destroyed some hybrids | No pre-zygotic isolation detected | 1983[84] |
| D. melanogaster | Disruptive selection | Pre-zygotic isolation; assortive mating; all later replications of the experiment failed | 1962[85] |
| D. melanogaster | N/A | N/A | 1997[86] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation | 1971[87] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation | 1973[88] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation | 1979[89] |
| Zea mays | Destroyed hybrids | Pre-zygotic isolation; reproductive character displacement | 1969[90] |
References
- ↑ Hannes Schuler, Glen R. Hood, Scott P. Egan, and Jeffrey L. Feder (2016), Meyers, Robert A, ed., "Modes and Mechanisms of Speciation", Reviews in Cell Biology and Molecular Medicine 2 (3): 60–93, doi:10.1002/3527600906, ISBN 9783527600908
- ↑ Jeremy L. Marshall, Michael L. Arnold, and Daniel J. Howard (2002), "Reinforcement: the road not taken", Trends in Ecology & Evolution 17 (12): 558–563, doi:10.1016/S0169-5347(02)02636-8
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 Jerry A. Coyne; H. Allen Orr (2004), Speciation, Sinauer Associates, pp. 1–545, ISBN 978-0-87893-091-3
- ↑ 4.0 4.1 Maria R. Servedio; Mohamed A. F. Noor (2003), "The Role of Reinforcement in Speciation: Theory and Data", Annual Review of Ecology, Evolution, and Systematics 34: 339–364, doi:10.1146/annurev.ecolsys.34.011802.132412
- ↑ 5.0 5.1 5.2 Daniel Ortíz-Barrientos, Alicia Grealy, and Patrik Nosil (2009), "The Genetics and Ecology of Reinforcement: Implications for the Evolution of Prezygotic Isolation in Sympatry and Beyond", Annals of the New York Academy of Sciences 1168: 156–182, doi:10.1111/j.1749-6632.2009.04919.x, PMID 19566707
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Daniel J. Howard (1993). Reinforcement: origin, dynamics and fate of an evolutionary hypothesis. In: Harrison, R. G. (eds) Hybrid Zones and the Evolutionary Process, Oxford University Press, pp. 46–69.
- ↑ 7.0 7.1 Mohamed A. F. Noor (1999), "Reinforcement and other consequences of sympatry", Heredity 83 (5): 503–508, doi:10.1038/sj.hdy.6886320, PMID 10620021
- ↑ 8.0 8.1 8.2 8.3 8.4 Glenn-Peter Sætre (2012). "Reinforcement". doi:10.1002/9780470015902.a0001754.pub3. ISBN 978-0470016176.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 Roger Butlin (1987), "Speciation by Reinforcement", Trends in Ecology & Evolution 2 (1): 8–13, doi:10.1016/0169-5347(87)90193-5, PMID 21227808
- ↑ Gerlinde Höbel and H. Carl Gerhardt (2003), "Reproductive character displacement in the acoustic communication system of green tree frogs (Hyla cinerea)", Evolution 57 (4): 894–904, doi:10.1554/0014-3820(2003)057[0894:RCDITA2.0.CO;2], PMID 12778558
- ↑ 11.0 11.1 David M. Hillis (1981), "Premating Isolating Mechanisms Among Three Species of the Rana pipiens Complex in Texas and Southern Oklahoma", Copeia 1981 (2): 312–319, doi:10.2307/1444220
- ↑ C. J. Schneider, M. Cunningham, and C. Moritz (1998), "Comparative phylogeography and the history of endemic vertebrates in the Wet Tropics rainforests of Australia", Molecular Ecology 7 (4): 487–498, doi:10.1046/j.1365-294x.1998.00334.x
- ↑ Conrad J. Hoskin, Megan Higgie, Keith R. McDonald, and Craig Moritz (2005), "Reinforcement drives rapid allopatric speciation", Nature 437 (7063): 1353–1356, doi:10.1038/nature04004, PMID 16251964, Bibcode: 2005Natur.437.1353H
- ↑ J. Michael Jones (1973), "Effects of thirty years hybridization on the toads Bufo americanus and Bufo woodhousii fowleri at Bloomington, Indian", Evolution 27 (3): 435–448, doi:10.1111/j.1558-5646.1973.tb00690.x, PMID 28564913
- ↑ Karin S. Pfennig (2003), "A test of alternative hypotheses for the evolution of reproductive isolation between spadefoot toads: Support for the reinforcement hypothesis", Evolution 57 (12): 2842–2851, doi:10.1554/03-228, PMID 14761062
- ↑ 16.0 16.1 16.2 Glenn-Peter Sætre, T. Moum, S. Bures, M. Kral, M. Adamjan, and J. Moreno (1997), "A sexually selected character displacement in flycatchers reinforces premating isolation", Nature 387 (6633): 589–592, doi:10.1038/42451, Bibcode: 1997Natur.387..589S
- ↑ R. V. Alatalo, L. Gustafsson, and A. Lundberg (1982), "Hybridization and breeding success of collared and pied flycatchers on the island of Gotland", Auk 99: 285–291
- ↑ Vallin, Niclas; Rice, Amber M.; Bailey, Richard I.; Husby, Arild; Qvarnström, Anna (April 2012). "Positive Feedback Between Ecological and Reproductive Character Displacement in a Young Avian Hybrid Zone" (in en). Evolution 66 (4): 1167–1179. doi:10.1111/j.1558-5646.2011.01518.x. PMID 22486696.
- ↑ Lars Wallin (1986), "Divergent character displacement in the song of two allospecies: the pied flycatcher Ficedula hypoleuca and the collared flycatcher Ficedula albicollis", Ibis 128 (2): 251–259, doi:10.1111/j.1474-919X.1986.tb02672.x
- ↑ L. M. Ratcliffe and Peter R. Grant (1983), "Species recognition in Darwin's finches (Geospiza, Gould). II. Geographic variation in mate preference", Animal Behaviour 31 (4): 1154–1165, doi:10.1016/S0003-3472(83)80022-0
- ↑ J. T. Ratti (1979). Reproductive Separation and Isolating Mechanisms between Sympatric Dark- and Light- Phase Western Grebes. American Ornithological Society, 93(3), 573–586.
- ↑ Emily J. Husdon and Trevor D. Price (2014), "Pervasive Reinforcement and the Role of Sexual Selection in Biological Speciation", Journal of Heredity 105: 821–833, doi:10.1093/jhered/esu041, PMID 25149257
- ↑ Mark J. Butler IV (1988), "Evaluation of Possible Reproductively Mediated Character Displacement in the Crayfishes, Orconectes rusticus and O. sanbornii", Ohio Journal of Science 88 (3): 87–91
- ↑ Edward C. Metz, Gerardo Gómez-Gutiérrez, and Victor D. Vacquier (1998), "Mitochondrial DNA and Bindin Gene Sequence Evolution Among Allopatric Species of the Sea Urchin Genus Arbacia", Molecular Biology and Evolution 15 (2): 185–195, doi:10.1093/oxfordjournals.molbev.a025914, PMID 9491615
- ↑ 25.0 25.1 25.2 Laura B. Geyer and Stephen R. Palumbi (2003), "Reproductive character displacement and the genetics of gamete recognition in tropical sea urchins", Evolution 57 (5): 1049–1060, doi:10.1554/0014-3820(2003)057[1049:RCDATG2.0.CO;2], PMID 12836822
- ↑ Howard D. Rundle (1998), "Reinforcement of stickleback mate preferences: Sympatry breeds contempt", Dolph Schluter 52 (1): 200–208, doi:10.1111/j.1558-5646.1998.tb05153.x, PMID 28568163
- ↑ C. Hubbs (1960), "Duration of sperm function in the percid fishes Etheostoma lepidum and Etheostoma spectabile, associated with sympatry of the parent populations", Copeia 1960 (1): 1–8, doi:10.2307/1439836
- ↑ 28.0 28.1 28.2 28.3 Jeremy R. Dettman, David J. Jacobson, Elizabeth Turner, Anne Pringle, and John W. Taylor (2003), "Reproductive isolation and phylogenetic divergence in Neurospora: Comparing methods of species recognition in a model eukaryote", Evolution 57 (12): 2721–2741, doi:10.1554/03-074, PMID 14761052
- ↑ Denson Kelly McLain and Karamjit S. Rai (1986), "Reinforcement for ethological isolation in the southeast asian Aedes albopictus subgroup (Diptera: Culicidae)", Evolution 40 (60): 1346–1350, doi:10.1111/j.1558-5646.1986.tb05759.x, PMID 28563509
- ↑ Patrik Nosil, Bernard J. Crespi, and Cristina P. Sandoval (2003), "Reproductive isolation driven by the combined effects of ecological adaptation and reinforcement", Proceedings of the Royal Society B 270 (1527): 1911–1918, doi:10.1098/rspb.2003.2457, PMID 14561304
- ↑ Patrik Nosil, Bernard J. Crespi, Regine Gries, and Gerhard Gries (2007), "Natural selection and divergence in mate preference during speciation", Genetica 129 (3): 309–327, doi:10.1007/s10709-006-0013-6, PMID 16900317
- ↑ 32.0 32.1 32.2 John R. Cooley, Chris Simon, David C. Marshall, Karen Slon, and Christopher Ehrhardt (2001), "Allochronic speciation, secondary contact, and reproductive character displacement in periodical cicadas (Hemiptera: Magicicada spp.): genetic, morphological, and behavioural evidence", Molecular Ecology 10 (3): 661–671, doi:10.1046/j.1365-294x.2001.01210.x, PMID 11298977
- ↑ Roger K. Butlin (1989). Reinforcement of premating isolation. In Otte, D. and Endler, John A. (eds) Speciation and its Consequences, Sinauer Associates, pp. 158–179, ISBN:0-87893-657-2
- ↑ J. H. Benedix Jr. and Daniel J. Howard (1991), "Calling song displacement in a zone of overlap and hybridization", Evolution 45 (8): 1751–1759, doi:10.1111/j.1558-5646.1991.tb02685.x, PMID 28563959
- ↑ Jonathan K. Waage (1975), "Reproductive Isolation and the Potential for Character Displacement in the Damselflies, Calopteryx Maculata and C. Aequabilis (Odonata: Calopterygidae)", Systematic Biology 24 (1): 24–36, doi:10.1093/sysbio/24.1.24
- ↑ Jonathan K. Waage (1979), "Reproductive character displacement in Calopteryx (Odonata: Calopterygidae", Evolution 33 (1): 104–116, doi:10.1111/j.1558-5646.1979.tb04667.x, PMID 28568062
- ↑ C. D. Jiggins (2006), "Reinforced butterfly speciation", Heredity 96 (2): 107–108, doi:10.1038/sj.hdy.6800754, PMID 16222327
- ↑ Vladimir A. Lukhtanov, Nikolai P. Kandul, Joshua B Plotkin, Alexander V. Dantchenko, David Haig, and Naomi E. Pierce (2005), "Reinforcement of prezygotic isolation and karyotype evolution in Agrodiaetus butterflies", Nature 436 (7049): 385–389, doi:10.1038/nature03704, PMID 16034417, Bibcode: 2005Natur.436..385L
- ↑ James Mallet (2006), "What does Drosophila genetics tell us about speciation?", Trends in Ecology & Evolution 21 (7): 386–393, doi:10.1016/j.tree.2006.05.004, PMID 16765478
- ↑ Lee Ehrman (1965), "Direct Observation of Sexual Isolation between Allopatric and between Sympatric Strains of the Different Drosophila paulistorum Races", Evolution 19 (4): 459–464, doi:10.2307/2406243
- ↑ Marvin Wasserman and H. Roberta Koepfer (1977), "Character displacement for sexual isolation between Drosophila mojavensis and Drosophila arizonensis", Evolution 31 (4): 812–823, doi:10.1111/j.1558-5646.1977.tb01073.x, PMID 28563708
- ↑ Mohamed A. F. Noor (1995), "Speciation driven by natural-selection in Drosophila", Nature 375 (6533): 674–675, doi:10.1038/375674a0, PMID 7791899, Bibcode: 1995Natur.375..674N
- ↑ Megan Higgie and Mark W. Blows (2007), "Are Traits That Experience Reinforcement Also Under Sexual Selection?", The American Naturalist 170 (3): 409–420, doi:10.1086/519401, PMID 17879191, http://espace.library.uq.edu.au/view/UQ:129790/UQ129790_OA.pdf
- ↑ Megan Higgie, Steve Chenoweth, and Mark W. Blows (2000), "Natural Selection and the Reinforcement of Mate Recognition", Science 290 (5491): 519–521, doi:10.1126/science.290.5491.519, PMID 11039933, Bibcode: 2000Sci...290..519H, https://espace.library.uq.edu.au/view/UQ:10714/Higgie_et_al_200.pdf
- ↑ Megan Higgie and Mark W. Blows (2008), "The Evolution of Reproductive Character Displacement Conflicts with how Sexual Selection Operates within a Species", Evolution 62 (5): 1192–1203, doi:10.1111/j.1558-5646.2008.00357.x, PMID 18298640
- ↑ Conrad J. Hoskin and Megan Higgie (2010), "Speciation via species interactions: the divergence of mating traits within species", Ecology Letters 13 (4): 409–420, doi:10.1111/j.1461-0248.2010.01448.x, PMID 20455922
- ↑ H. MacCarley (1964), "Ethological isolation in the cenospecies Peromyscus leucopus", Evolution 18 (2): 331–342, doi:10.1111/j.1558-5646.1964.tb01605.x
- ↑ 48.0 48.1 48.2 Michael S. Johnson (1982), "Polymorphism for direction of coil in Partula suturalis: Behavioral isolation and positive frequency dependent selection", Heredity 49 (2): 145–151, doi:10.1038/hdy.1982.80
- ↑ J. Murray and B. Clarke (1980), "The genus Partula on Moorea: speciation in progress", Proceedings of the Royal Society B 211 (1182): 83–117, doi:10.1098/rspb.1980.0159, Bibcode: 1980RSPSB.211...83M
- ↑ Carl T. Bergstrom and Lee Alan Dugatkin (2016), Evolution (2nd ed.), W. W. Norton & Company, pp. 508–509, ISBN 9780393937930
- ↑ Yuichi Kameda, Atsushi Kawakita, and Makoto Kato (2009), "Reproductive Character Displacement in Genital Morphology in Satsuma Land Snails", The American Naturalist 173 (5): 689–697, doi:10.1086/597607, PMID 19298185
- ↑ Esther B. Wullschleger, Jürgen Wiehn, and Jukka Jokela (2002), "Reproductive character displacement between the closely related freshwater snails Lymnaea peregra and L. ovata", Evolutionary Ecology Research 4: 247–257
- ↑ Y. H. Lee, T. Ota, and V. D. Vacquier (1995), "Positive selection is a general phenomenon in the evolution of abalone sperm lysin", Molecular Biology and Evolution 12 (2): 231–238, doi:10.1093/oxfordjournals.molbev.a040200, PMID 7700151
- ↑ Robin Hopkins (2013), "Reinforcement in plants", New Phytologist 197 (4): 1095–1103, doi:10.1111/nph.12119, PMID 23495388
- ↑ 55.0 55.1 Robin Hopkins and Mark D. Rausher (2011), "Identification of two genes causing reinforcement in the Texas wildflower Phlox drummondii", Nature 469 (7330): 411–414, doi:10.1038/nature09641, PMID 21217687, Bibcode: 2011Natur.469..411H
- ↑ Rob Hopkins, Rafael F. Guerrero, Mark D. Rausher, and Mark Kirkpartrick (2014), "Strong Reinforcing Selection in a Texas Wildflower", Current Biology 24 (17): 1995–1999, doi:10.1016/j.cub.2014.07.027, PMID 25155503
- ↑ Donald A. Levin and Harold W. Kerster (1967), "Natural selection for reproductive isolation in Phlox", Evolution 21 (4): 679–687, doi:10.1111/j.1558-5646.1967.tb03425.x, PMID 28563087
- ↑ Michael D. Whalen (1978), "Reproductive Character Displacement and Floral Diversity in Solanum Section Androceras", Systematic Biology 3 (1): 77–86, doi:10.2307/2418533
- ↑ Constance I. Millar (1983), "A steep cline in Pinus muricata", Evolution 37 (2): 311–319, doi:10.1111/j.1558-5646.1983.tb05541.x, PMID 28568365
- ↑ 60.0 60.1 Thomas McNeilly and Janis Antonovics (1968), "Evolution in closely adjacent plant populations. IV. Barriers to gene flow", Heredity 23 (2): 205–218, doi:10.1038/hdy.1968.29
- ↑ Verne Grant (1966), "The selective origin of incompatibility barriers in the plant genus Gilia", The American Naturalist 100 (911): 99–118, doi:10.1086/282404
- ↑ Douglas W. Schemske (1981), "Floral convergence and pollinator sharing in two bee-pollinated tropical herbs", Ecology 62 (4): 946–954, doi:10.2307/1936993
- ↑ Kathleen M. Kay and Douglas W. Schemske (2003), "Pollinator assemblages and visitation rates for 11 species of neotropical Costus (Costaceae)", Biotropica 35 (2): 198–207, doi:10.1646/02159
- ↑ Kathleen M. Kay and Douglas W. Schemske (2008), "Natural selection reinforces speciation in a radiation of neotropical rainforest plants", Evolution 62 (10): 2628–2642, doi:10.1111/j.1558-5646.2008.00463.x, PMID 18637960
- ↑ Stefan G Michalski and Walter Durka (2015), "Separation in flowering time contributes to the maintenance of sympatric cryptic plant lineages", Ecology and Evolution 5 (11): 2172–2184, doi:10.1002/ece3.1481, PMID 26078854
- ↑ Rebecca S. Taylor and Vicki L. Friesen (2017), "The role of allochrony in speciation", Molecular Ecology 26 (13): 3330–3342, doi:10.1111/mec.14126, PMID 28370658
- ↑ Jerry A. Coyne and H. Allen Orr (1997), ""Patterns of Speciation in Drosophila" Revisited", Evolution 51 (1): 295–303, doi:10.1111/j.1558-5646.1997.tb02412.x, PMID 28568795
- ↑ A. R. McCune and N. R. Lovejoy. (1998). The relative rate of sympatric and allopatric speciation in fishes. In D. J. Howard and S. H. Berlocher (eds) Endless Forms: Species and Speciation, Oxford University Press, pp. 172–185.
- ↑ Leonie C. Moyle, Matthew S Olson, and Peter Tiffin (2004), "Patterns of reproductive isolation in three angiosperm genera", Evolution 58 (6): 1195–1208, doi:10.1554/03-511, PMID 15266970
- ↑ 70.0 70.1 William R. Rice and Ellen E. Hostert (1993), "Laboratory Experiments on Speciation: What Have We Learned in 40 Years?", Evolution 47 (6): 1637–1653, doi:10.1111/j.1558-5646.1993.tb01257.x, PMID 28568007
- ↑ T. Dobzhansky, O. Pavlovsky, and J. R. Powell (1976), "Partially Successful Attempt to Enhance Reproductive Isolation Between Semispecies of Drosophila paulistorum", Evolution 30 (2): 201–212, doi:10.2307/2407696, PMID 28563045
- ↑ Karl F. Koopman (1950), "Natural Selection for Reproductive Isolation Between Drosophila pseudoobscura and Drosophila persimilis", Evolution 4 (2): 135–148, doi:10.2307/2405390
- ↑ Stella A. Crossley (1974), "Changes in Mating Behavior Produced by Selection for Ethological Isolation Between Ebony and Vestigial Mutants of Drosophila melanogaster", Evolution 28 (4): 631–647, doi:10.1111/j.1558-5646.1974.tb00795.x, PMID 28564833
- ↑ G. R. Knight (1956), "Selection for sexual isolation within a species", Evolution 10: 14–22, doi:10.1111/j.1558-5646.1956.tb02825.x
- ↑ A. Fukatami and D. Moriwaki (1970), "Selection for sexual isolation in Drosophila melanogaster by a modification of Koopman's method", The Japanese Journal of Genetics 45 (3): 193–204, doi:10.1266/jjg.45.193
- ↑ Wallace B (1953). "Genetic divergence of isolated populations of Drosophila melanogaster". International Congress of Genetics, Proceedings 9: 761–764.
- ↑ J. S. F. Barker and L. J. E. Karlsson (1974), "Effects of population size and selection intensity on responses to disruptive selection in Drosophila melanogaster", Genetics 78 (2): 715–735, doi:10.2307/2407287, PMID 4217303
- ↑ B. Wallace (1982), "Drosophila melanogaster populations selected for resistances to NaCl and CuSO4 in both allopatry and sympatry", Journal of Heredity 73 (1): 35–42, doi:10.1093/oxfordjournals.jhered.a109572, PMID 6802898
- ↑ Lee Ehrman, Marney A. White, and B. Wallace. 1991. A long-term study involving Drosophila melanogaster and toxic media. Pp. 175-209 in M. K. Hecht, B. Wallace, and R. J. Maclntyre, eds. Evolutionary biology, vol. 25. Plenum Press, New York.
- ↑ Forbes W. Robertson (1966), "A test of sexual isolation in Drosophila", Genetical Research 8 (2): 181–187, doi:10.1017/S001667230001003X, PMID 5922518
- ↑ Forbes W. Robertson (1966), "The ecological genetics of growth in Drosophila 8. Adaptation to a New Diet", Genetical Research 8 (2): 165–179, doi:10.1017/S0016672300010028, PMID 5922517
- ↑ B. S. Grant and L. E. Mettler (1969), "Disruptive and stabilizing selection on the" escape" behavior of Drosophila melanogaster", Genetics 62 (3): 625–637, doi:10.1093/genetics/62.3.625, PMID 17248452
- ↑ E. B. Spiess and C. M. Wilke (1984), "Still another attempt to achieve assortive mating by disruptive selection in Drosophila", Evolution 38 (3): 505–515, doi:10.1111/j.1558-5646.1984.tb00316.x, PMID 28555983
- ↑ A. A. Harper and D. M. Lambert (1983), "The population genetics of reinforcing selection", Genetica 62 (1): 15–23, doi:10.1007/BF00123305
- ↑ J. M. Thoday and J. B. Gibson (1962), "Isolation by disruptive selection", Nature 193 (4821): 1164–1166, doi:10.1038/1931164a0, PMID 13920720, Bibcode: 1962Natur.193.1164T
- ↑ Ellen E. Hostert (1997), "Reinforcement: a new perspective on an old controversy", Evolution 51 (3): 697–702, doi:10.1111/j.1558-5646.1997.tb03653.x, PMID 28568598
- ↑ Lee Ehrman (1971), "Natural selection and the origin of reproductive isolation", American Naturalist 105 (945): 479–483, doi:10.1086/282739
- ↑ Lee Ehrman (1973), "More on natural selection and the origin of reproductive isolation", American Naturalist 107 (954): 318–319, doi:10.1086/282835
- ↑ Lee Ehrman (1979), "Still more on natural selection and the origin of reproductive isolation", American Naturalist 113: 148–150, doi:10.1086/283371
- ↑ E. Paterniani (1969), "Selection for Reproductive Isolation between Two Populations of Maize, Zea mays L", Evolution 23 (4): 534–547, doi:10.1111/j.1558-5646.1969.tb03539.x, PMID 28562870
 |






