Chemistry:Alteplase
| Clinical data | |
|---|---|
| Trade names | Activase, Actilyse, Cathflo Activase, others |
| Other names | t-PA, rt-PA |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C2569H3928N746O781S40 |
| Molar mass | 59042.52 g·mol−1 |
| (verify) | |
Alteplase (t-PA), a biosynthetic form of human tissue-type plasminogen activator (t-PA), is a thrombolytic medication, used to treat acute ischemic stroke, acute ST-elevation myocardial infarction (a type of heart attack), pulmonary embolism associated with low blood pressure, and blocked central venous catheter.[4] It is given by injection into a vein or artery.[4] Alteplase is the same as the normal human plasminogen activator produced in vascular endothelial cells[5] and is synthesized via recombinant DNA technology in Chinese hamster ovary cells (CHO). Alteplase causes the breakdown of a clot by inducing fibrinolysis.[6]
It is on the World Health Organization's List of Essential Medicines.[7]
Medical uses
Alteplase is mainly used to treat acute ischemic stroke, acute myocardial infarction, acute massive pulmonary embolism, and blocked catheters.[4][1][2] Similar to other thrombolytic drugs, alteplase is used to dissolve clots to restore tissue perfusion, but this can vary depending on the pathology.[8][9][10] Generally, alteplase is delivered intravenously into the body.[6] To treat blocked catheters, alteplase is administered directly into the catheter.[6]
Alteplase has also been used off-label for deep vein thrombosis, peripheral artery disease, pleural effusion in children, prosthetic valve thrombosis, frostbite, and peritonitis.[11][12]
Ischemic stroke
In adults diagnosed with acute ischemic stroke, thrombolytic treatment with alteplase is the standard of care during early management (within 4.5 hours of symptom onset).[9] When mechanical thrombectomy is not available, alteplase may be considered up to 9 hours after symptom onset.[13]
Administration of alteplase is associated with improved functional outcomes and reduced incidence of disability.[11] Alteplase used in conjunction with mechanical thrombectomy is associated with better outcomes.[14] Use is restricted, however, if there is a risk of major bleeding or if there may be another cause of stroke symptoms.[9][15] Alteplase is not recommended for those with non-disabling stroke.[13]
For patients with early recurrent ischemic stroke, repeated use of alteplase may be safe and efficacious.[16]
Alteplase is also commonly used in children, though guidelines are not yet standardized as they are for adults.[17][18]
Myocardial infarction
Currently, the preferred treatment for ST-elevation myocardial infarction (STEMI) is percutaneous coronary intervention (PCI).[8] However, PCI is only available at 25% of hospitals in the United States; alteplase is recommended if the patient is at a non-PCI capable hospital and cannot be transferred to receive PCI in under 120 minutes.[8][19] Alteplase can also be used before arriving at the hospital if transportation time is anticipated to be greater than 30 minutes.[20]
Alteplase may be used in conjunction with aspirin and heparin.[11] An accelerated infusion of alteplase was found to significantly reduce mortality in comparison to a non-accelerated infusion, though it also slightly increases the risk of major bleeding.[21]
Alteplase should not be used in cases of acute coronary syndrome other than STEMI.[20]
Pulmonary embolism
As of 2019, alteplase is the most commonly used medication to treat pulmonary embolism (PE).[22] Alteplase has a short infusion time of 2 hours and a half-life of 4–6 minutes.[22] Alteplase has been approved by the FDA, and treatment can be done via systemic thrombolysis or catheter-directed thrombolysis.[22][23]
Systemic thrombolysis can quickly restore right ventricular function, heart rate, and blood pressure in patients with acute PE.[24] However, standard doses of alteplase used in systemic thrombolysis may lead to massive bleeding, such as intracranial hemorrhage, particularly in older patients.[22] A systematic review has shown that low-dose alteplase is safer than and as effective as the standard amount.[25]
Catheter-directed thrombolysis may be more efficient than systemic thrombolysis, as alteplase is locally administered to the occlusion site, and wash-away of the medication into other blood vessels is minimized.[24] This procedure involves positioning a multi-sidehole catheter into the blood clot.[24]
Alteplase may be used to treat PE if patients have a high risk for complications, such as if:[26]
- they are hypotensive with a systolic blood pressure less than 90 mmHg[27][28]
- they are in cardiac arrest presumed to be caused by a pulmonary embolism [27]
- their clinical exam shows signs of deterioration or worsening of symptoms[28]
Blocked catheters
Alteplase can be used in small doses to clear blood clots that obstruct a catheter, reopening the catheter so it can continue to be used.[2][11] Catheter obstruction is commonly observed with a central venous catheter.[29] Currently, the standard treatment for catheter obstructions in the United States is alteplase administration.[5] Alteplase is effective and low risk for treating blocked catheters in adults and children.[5][29] Overall, adverse effects of alteplase for clearing blood clots are rare.[30] Novel alternatives to treat catheter occlusion, such as tenecteplase, reteplase, and recombinant urokinase, offer the advantage of shorter dwell times than alteplase.[29]
Contraindications
A person should not receive alteplase treatment if testing shows they are not suffering from an acute ischemic stroke or if the risks of treatment outweigh the likely benefits.[9] Alteplase is contraindicated in those with bleeding disorders that increase a person's tendency to bleed and in those with an abnormally low platelet count.[15] Active internal bleeding and high blood pressure are additional contraindications for alteplase.[15] The safety of alteplase in the pediatric population has not been determined definitively.[15] Additional contraindications for alteplase when used specifically for acute ischemic stroke include current intracranial hemorrhage and subarachnoid hemorrhage.[31] Contraindications for use of alteplase in people with a STEMI are similar to those of acute ischemic stroke.[8] People with an acute ischemic stroke may also receive other therapies including mechanical thrombectomy.[9]
Adverse effects
Given that alteplase is a thrombolytic medication, a common adverse effect is bleeding, which can be life threatening.[32] Adverse effects of alteplase include symptomatic intracranial hemorrhage and fatal intracranial hemorrhage.[32]
Angioedema is another adverse effect of alteplase, which can be life-threatening if the airway becomes obstructed.[1] Other side effects may rarely include allergic reactions.[4]
Mechanism of action
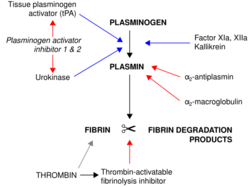
Alteplase binds to fibrin in a blood clot and activates the clot-bound plasminogen.[6] Alteplase cleaves plasminogen at the site of its Arg561-Val562 peptide bond to form plasmin.[6] Plasmin is a fibrinolytic enzyme that cleaves the cross-links between polymerized fibrin molecules, causing the blood clot to break down and dissolve, a process called fibrinolysis.[6]
Regulation and inhibition
Plasminogen activator inhibitor 1 stops alteplase activity by binding to it and forming an inactive complex, which is removed from the bloodstream by the liver.[6] Fibrinolysis by plasmin is extremely short-lived due to plasmin inhibitors, which inactivate and regulate plasmin activity.[6]
Society and culture
Alteplase was added to the World Health Organization's List of Essential Medicines in 2019, for use in ischemic stroke.[33][34]
As early use of alteplase is important for ischemic stroke, treatment delay is a serious concern.[35] Many reasons for delay exist, including lack of access to healthcare, late presentation, late assessment, misdiagnosis, and management of comorbidities.[35]
Alteplase is extremely underused in low- and middle-income countries.[36] This may be due to its high cost and the fact that it is often not covered by health insurance.[36]
There may be citation bias in the literature on alteplase in ischemic stroke, as studies reporting positive results for tissue plasminogen activator are more likely to be cited in following studies than those reporting negative or neutral results.[37]
There is a sex difference in the use of intravenous tissue plasminogen activator, as it is less likely to be used for women with acute ischemic stroke than men.[38] However, this difference has been improving since 2008.[38]
Economics
The cost of alteplase in the United States increased by 111% between 2005 and 2014, despite there being no proportional increase in the costs of other prescription drugs.[39] However, alteplase continues to be cost-effective.[39]
Brand names
Alteplase is marketed as Actilyse, Activase, and Cathflo or Cathflo Activase.[40][41]
History
FDA approval and paradigm shift
Alteplase was approved for medical use in the United States in November 1987 for the treatment of myocardial infarction.[4][1][42][43] This was just seven years after the first efforts were made to produce recombinant t-PA, making it one of the fastest drug developments in history.[43]
In 1995, a study by the National Institute of Neurological Disorders and Stroke showed the effectiveness of administering intravenous alteplase to treat ischemic stroke.[44] This sparked a medical paradigm shift as it redesigned stroke treatment in the emergency department to allow for timely assessment and therapy for ischemic stroke patients.[44]
Commercialization
Commercialization and large scale manufacture of human t-PA has been made possible through the generation of Chinese hamster ovary cells, which are capable of producing alteplase (recombinant human t-PA) with the use of recombinant DNA technology.[40] Brands include Activase and Cathflo Activase, marketed by Genentech Inc. in the US, and Actilyse, marketed by Boehringer Ingelheim in Germany.[2][40]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 "Activase- alteplase kit". 5 December 2018. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c669f77c-fa48-478b-a14b-80b20a0139c2.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 "Cathflo Activase- alteplase injection, powder, lyophilized, for solution". 6 September 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=91ecdef2-95ff-42dd-a31c-c8a09cab3ad9.
- ↑ "Actilyse". https://www.ema.europa.eu/en/medicines/human/referrals/actilyse.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 "Alteplase Monograph for Professionals". https://www.drugs.com/monograph/alteplase.html.
- ↑ Jump up to: 5.0 5.1 5.2 "Management of occlusion and thrombosis associated with long-term indwelling central venous catheters". Lancet 374 (9684): 159–69. July 2009. doi:10.1016/S0140-6736(09)60220-8. PMID 19595350.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 "Tissue Plasminogen Activator". StatPearls. Treasure Island (FL): StatPearls Publishing. April 2020. http://www.ncbi.nlm.nih.gov/books/NBK507917/. Retrieved 2020-11-10.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 "2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines". Circulation 127 (4): e362-425. January 2013. doi:10.1161/CIR.0b013e3182742cf6. PMID 23247304.
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 "Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association". Stroke 50 (12): e344–e418. December 2019. doi:10.1161/STR.0000000000000211. PMID 31662037.
- ↑ "2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association". Stroke 49 (3): e46–e110. March 2018. doi:10.1161/STR.0000000000000158. PMID 29367334.
- ↑ Jump up to: 11.0 11.1 11.2 11.3 "Alteplase". StatPearls. Treasure Island (FL): StatPearls Publishing. 2020. http://www.ncbi.nlm.nih.gov/books/NBK499977/. Retrieved 2020-10-30.
- ↑ TPA Therapy. Treasure Island: StatPearls. 2020.
- ↑ Jump up to: 13.0 13.1 Solomon, Caren G., ed (July 2020). "Acute Ischemic Stroke". The New England Journal of Medicine 383 (3): 252–260. doi:10.1056/NEJMcp1917030. PMID 32668115.
- ↑ "Mechanical Thrombectomy Outcomes With and Without Intravenous Thrombolysis in Stroke Patients: A Meta-Analysis". Stroke 48 (9): 2450–2456. September 2017. doi:10.1161/STROKEAHA.117.017320. PMID 28747462.
- ↑ Jump up to: 15.0 15.1 15.2 15.3 "Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association". Stroke 47 (2): 581–641. February 2016. doi:10.1161/STR.0000000000000086. PMID 26696642.
- ↑ "Safety and Efficacy of Repeated Thrombolysis with Alteplase in Early Recurrent Ischemic Stroke: A Systematic Review". Journal of Stroke and Cerebrovascular Diseases 28 (10): 104290. October 2019. doi:10.1016/j.jstrokecerebrovasdis.2019.07.006. PMID 31371140.
- ↑ "Pediatric Thrombolysis: A Practical Approach". Frontiers in Pediatrics 5: 260. December 2017. doi:10.3389/fped.2017.00260. PMID 29270396.
- ↑ "Thrombolysis in pediatric stroke study". Stroke 46 (3): 880–5. March 2015. doi:10.1161/STROKEAHA.114.008210. PMID 25613306.
- ↑ "Recombinant tissue plasminogen activators (rtPA): a review". Clinical Pharmacology and Therapeutics 97 (3): 274–85. March 2015. doi:10.1002/cpt.33. PMID 25670034.
- ↑ Jump up to: 20.0 20.1 "Thrombolytics for Acute Myocardial Infarction in a Pre-Hospital Setting: A Review of Comparative Safety and Guidelines". CADTH. July 2019. https://www.cadth.ca/sites/default/files/pdf/htis/2019/RC1144%20Final.pdf. Retrieved 12 November 2020.
- ↑ "Comparative efficacy and safety of reperfusion therapy with fibrinolytic agents in patients with ST-segment elevation myocardial infarction: a systematic review and network meta-analysis". Lancet 390 (10096): 747–759. August 2017. doi:10.1016/s0140-6736(17)31441-1. PMID 28831992.
- ↑ Jump up to: 22.0 22.1 22.2 22.3 "Update on Thrombolytic Therapy in Acute Pulmonary Thromboembolism". The Eurasian Journal of Medicine 51 (2): 186–190. June 2019. doi:10.5152/eurasianjmed.2019.19291. PMID 31258361.
- ↑ "Systemic Thrombolysis for Pulmonary Embolism: A Review". P & T 41 (12): 770–775. December 2016. PMID 27990080.
- ↑ Jump up to: 24.0 24.1 24.2 "Ultrasound-assisted thrombolysis for acute pulmonary embolism: a systematic review". European Heart Journal 35 (12): 758–64. March 2014. doi:10.1093/eurheartj/ehu029. PMID 24497337.
- ↑ "Lower dosage of recombinant tissue-type plasminogen activator (rt-PA) in the treatment of acute pulmonary embolism: a systematic review and meta-analysis". Thrombosis Research 133 (3): 357–63. March 2014. doi:10.1016/j.thromres.2013.12.026. PMID 24412030.
- ↑ "Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials". Circulation 110 (6): 744–9. August 2004. doi:10.1161/01.CIR.0000137826.09715.9C. PMID 15262836.
- ↑ Jump up to: 27.0 27.1 "Management of PE". January 2020. http://www.acc.org/latest-in-cardiology/articles/2020/01/27/07/42/management-of-pe.
- ↑ Jump up to: 28.0 28.1 "Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report". Chest 149 (2): 315–352. February 2016. doi:10.1016/j.chest.2015.11.026. PMID 26867832.
- ↑ Jump up to: 29.0 29.1 29.2 "Thrombolytic therapy for central venous catheter occlusion". Haematologica 97 (5): 641–50. May 2012. doi:10.3324/haematol.2011.050492. PMID 22180420.
- ↑ "Efficacy, safety, and cost of thrombolytic agents for the management of dysfunctional hemodialysis catheters: a systematic review". Pharmacotherapy 31 (10): 1031–40. October 2011. doi:10.1592/phco.31.10.1031. PMID 21950645.
- ↑ "Changing contraindications for t-PA in acute stroke: review of 20 years since NINDS". Current Cardiology Reports 17 (10): 81. October 2015. doi:10.1007/s11886-015-0633-5. PMID 26277361.
- ↑ Jump up to: 32.0 32.1 "Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials". Lancet 384 (9958): 1929–35. November 2014. doi:10.1016/S0140-6736(14)60584-5. PMID 25106063.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Executive summary: the selection and use of essential medicines 2019: report of the 22nd WHO Expert Committee on the selection and use of essential medicines. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.05. License: CC BY-NC-SA 3.0 IGO.
- ↑ Jump up to: 35.0 35.1 "Factors delaying intravenous thrombolytic therapy in acute ischaemic stroke: a systematic review of the literature". Journal of Neurology 268 (8): 2723–2734. March 2020. doi:10.1007/s00415-020-09803-6. PMID 32206899.
- ↑ Jump up to: 36.0 36.1 "Presentation, Evaluation, Management, and Outcomes of Acute Stroke in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis". Neuroepidemiology 51 (1–2): 104–112. 2018. doi:10.1159/000491442. PMID 30025394.
- ↑ "Citation bias favoring positive clinical trials of thrombolytics for acute ischemic stroke: a cross-sectional analysis". Trials 17 (1): 473. September 2016. doi:10.1186/s13063-016-1595-7. PMID 27677444.
- ↑ Jump up to: 38.0 38.1 "Sex differences in IV thrombolysis treatment for acute ischemic stroke: A systematic review and meta-analysis". Neurology 95 (1): e11–e22. July 2020. doi:10.1212/wnl.0000000000009733. PMID 32522796.
- ↑ Jump up to: 39.0 39.1 "Cost of Alteplase Has More Than Doubled Over the Past Decade". Stroke 48 (7): 2000–2002. July 2017. doi:10.1161/strokeaha.116.015822. PMID 28536176.
- ↑ Jump up to: 40.0 40.1 40.2 "Tissue-type plasminogen activator: a historical perspective and personal account". Journal of Thrombosis and Haemostasis 2 (4): 541–6. April 2004. doi:10.1111/j.1538-7933.2004.00645.x. PMID 15102005.
- ↑ "Cathflo Activase Uses, Side Effects & Warnings". https://www.drugs.com/mtm/cathflo-activase.html.
- ↑ "Activase: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=103172.
- ↑ Jump up to: 43.0 43.1 "The tissue-type plasminogen activator story". Arteriosclerosis, Thrombosis, and Vascular Biology 29 (8): 1151–5. August 2009. doi:10.1161/ATVBAHA.108.179655. PMID 19605778.
- ↑ Jump up to: 44.0 44.1 "Twenty-Year History of the Evolution of Stroke Thrombolysis With Intravenous Alteplase to Reduce Long-Term Disability". Stroke 46 (8): 2341–6. August 2015. doi:10.1161/STROKEAHA.114.007564. PMID 26152294.
Further reading
- Australian Public Assessment Report for Alteplase (AusPAR) (Report). February 2011. https://www.tga.gov.au/sites/default/files/auspar-actilyse.pdf.
External links
- "Alteplase". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/alteplase.