Chemistry:Deoxyguanosine
From HandWiki
| |
| |
| Names | |
|---|---|
| IUPAC name
2-Amino-9-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-3H-purin-6-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | Deoxyguanosine |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H13N5O4 | |
| Molar mass | 267.245 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
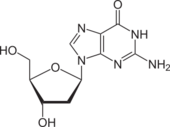
Deoxyguanosine is composed of the purine nucleobase guanine linked by its N9 nitrogen to the C1 carbon of deoxyribose. It is similar to guanosine, but with one hydroxyl group removed from the 2' position of the ribose sugar (making it deoxyribose). If a phosphate group is attached at the 5' position, it becomes deoxyguanosine monophosphate.
Deoxyguanosine is one of the four deoxyribonucleosides that make up DNA.[2]
See also
References
- ↑ "2'-deoxyguanosine". PubChem Compound Database. National Center for Biotechnology Information. 24 December 2016. https://pubchem.ncbi.nlm.nih.gov/compound/2_-deoxyguanosine#section=Top.
- ↑ "Human Metabolome Database (HMDB) metabocard for Deoxyguanosine (HMDB0000085)". http://www.hmdb.ca/metabolites/HMDB0000085.
 |

