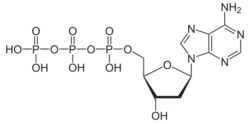
Chemistry:Deoxyadenosine triphosphate
| |
| |
| Names | |
|---|---|
| IUPAC name
[[(2R,3S,5R)-5-(6-aminopurin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] phosphono hydrogen phosphate
| |
| Other names
dATP, 2'-deoxyadenosine triphosphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C10H16N5O12P3 | |
| Molar mass | 491.181623 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Deoxyadenosine triphosphate (dATP) is a nucleotide used in cells for DNA synthesis (or replication), as a substrate of DNA polymerase.[1]
Deoxyadenosine triphosphate is produced from DNA by the action of nuclease P1, adenylate kinase, and pyruvate kinase.[2]
Health effects
High levels of dATP can be toxic and result in impaired immune function, since dATP acts as a noncompetitive inhibitor for the DNA synthesis enzyme ribonucleotide reductase. Patients with adenosine deaminase deficiency (ADA) tend to have elevated intracellular dATP concentrations because adenosine deaminase normally curbs adenosine levels by converting it into inosine.[3][4] Deficiency of this deaminase also causes immunodeficiency.[5]
In cardiac myosin, dATP is an alternative to ATP as an energy substrate for facilitating cross-bridge formation.[6][7]
See also
- Adenosine triphosphate (ATP)
- Adenosine deaminase deficiency (ADA)
- Dilated cardiomyopathy (DCM)
References
- ↑ "A study of the mechanism of T4 DNA polymerase with diastereomeric phosphorothioate analogues of deoxyadenosine triphosphate". The Journal of Biological Chemistry 257 (13): 7684–7688. July 1982. doi:10.1016/S0021-9258(18)34435-1. PMID 7045112.
- ↑ "Enzymic synthesis of deoxyATP using DNA as starting material". The Journal of Organic Chemistry 50 (7): 1076–1079. 1985-04-01. doi:10.1021/jo00207a032. ISSN 0022-3263.
- ↑ "Effects of deoxyadenosine triphosphate and 9-beta-D-arabinofuranosyl-adenine 5'-triphosphate on human ribonucleotide reductase from Molt-4F cells and the concept of "self-potentiation"". Cancer Research 40 (10): 3555–3558. October 1980. PMID 6159965. https://cancerres.aacrjournals.org/content/40/10/3555.
- ↑ "Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency". Proceedings of the National Academy of Sciences of the United States of America 75 (1): 472–476. January 1978. doi:10.1073/pnas.75.1.472. PMID 272665. Bibcode: 1978PNAS...75..472C.
- ↑ "Carrier frequency of a nonsense mutation in the adenosine deaminase (ADA) gene implies a high incidence of ADA-deficient severe combined immunodeficiency (SCID) in Somalia and a single, common haplotype indicates common ancestry". Annals of Human Genetics 71 (Pt 3): 336–347. May 2007. doi:10.1111/j.1469-1809.2006.00338.x. PMID 17181544.
- ↑ "2-Deoxyadenosine triphosphate restores the contractile function of cardiac myofibril from adult dogs with naturally occurring dilated cardiomyopathy". American Journal of Physiology. Heart and Circulatory Physiology 310 (1): H80–H91. January 2016. doi:10.1152/ajpheart.00530.2015. PMID 26497964.
- ↑ "Cardiac myosin activation with 2-deoxy-ATP via increased electrostatic interactions with actin". Proceedings of the National Academy of Sciences of the United States of America 116 (23): 11502–11507. June 2019. doi:10.1073/pnas.1905028116. PMID 31110001. Bibcode: 2019PNAS..11611502P.
Further reading
- "Deoxyadenosine metabolism and cytotoxicity in cultured mouse T lymphoma cells: a model for immunodeficiency disease" (in English). Cell 14 (2): 365–375. June 1978. doi:10.1016/0092-8674(78)90122-8. PMID 208780.
- "Restoration of adenosine deaminase-deficient human thymocyte development in vitro by inhibition of deoxynucleoside kinases". Journal of Immunology 181 (11): 8153–8161. December 2008. doi:10.4049/jimmunol.181.11.8153. PMID 19018008.
- "Deoxycytidine therapy in two patients with adenosine deaminase deficiency and severe immunodeficiency disease". Clinical Immunology and Immunopathology 37 (1): 30–36. October 1985. doi:10.1016/0090-1229(85)90132-1. PMID 3161676.
- "Deoxyadenosine triphosphate acting as an energy-transferring molecule in adenosine deaminase inhibited human erythrocytes". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1094 (3): 257–262. September 1991. doi:10.1016/0167-4889(91)90084-b. PMID 1911876.
External links
 |

