Chemistry:Guanosine diphosphate
| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H15N5O11P2 | |
| Molar mass | 443.200522 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
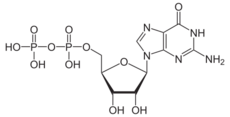
Guanosine diphosphate, abbreviated GDP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside guanosine. GDP consists of a pyrophosphate group, a pentose sugar ribose, and the nucleobase guanine.[1]
GDP is the product of GTP dephosphorylation by GTPases, e.g., the G-proteins that are involved in signal transduction.
GDP is converted into GTP with the help of pyruvate kinase and phosphoenolpyruvate.
GDP and GTP
Hydrolysis of GTP into GDP
The hydrolysis of GTP to GDP is facilitated by GTPase enzymes, which utilize a conserved active site motif known as the GTPase-activating protein (GAP). Initially, a water molecule is coordinated by the active site residues of the GTPase enzyme. The water molecule attacks the γ-phosphate of GTP, leading to the formation of a pentavalent transition state. This transition state is stabilized by interactions with the active site residues, including conserved catalytic residues. As a result, the γ-phosphate is cleaved, and inorganic phosphate (Pi) is released. This step also causes a conformational change in the enzyme that promotes the release of GDP.[2]
Biochemical functions
Intracellular signaling
GDP is involved in intracellular signaling processes functioning as a critical regulator in the activity of GTPases. GTPases act as molecular switches, cycling between an active GTP-bound state and an inactive GDP-bound state. The interconversion between GDP and GTP is tightly controlled and serves as a molecular timer for signal transduction pathways. When an extracellular signal triggers the activation of a G-protein coupled receptor (GPCR), the associated G-protein exchanges its bound GDP for GTP, leading to a conformational change and activation of downstream signaling cascades.[3] This activation can stimulate a variety of cellular responses, including modulation of gene expression, cytoskeletal rearrangements, and regulation of enzymatic activities. The hydrolysis of GTP to GDP by the GTPase activity of the G-protein restoring the inactive state, thus terminates the signaling event.[4]
See also
References
- ↑ Crane, Laura J; Miller, David Lee (1974). "Guanosine triphosphate and guanosine diphosphate as conformation-determining molecules. Differential interaction of a fluorescent probe with the guanosine nucleotide complexes of bacterial elongation factor Tu". Biochemistry 13 (5): 933–939. doi:10.1021/bi00702a017. PMID 4591619.
- ↑ Calixto, Ana R.; Moreira, Cátia; Pabis, Anna; Kötting, Carsten; Gerwert, Klaus; Rudack, Till; Kamerlin, Shina C.L. (2019-07-10). "GTP Hydrolysis Without an Active Site Base: A Unifying Mechanism for Ras and Related GTPases" (in en). Journal of the American Chemical Society 141 (27): 10684–10701. doi:10.1021/jacs.9b03193. ISSN 0002-7863. https://pubs.acs.org/doi/10.1021/jacs.9b03193.
- ↑ Downes, G. B.; Gautam, N. (1999-12-15). "The G protein subunit gene families". Genomics 62 (3): 544–552. doi:10.1006/geno.1999.5992. ISSN 0888-7543. PMID 10644457. https://pubmed.ncbi.nlm.nih.gov/10644457/.
- ↑ Schmidt, Anja; Hall, Alan (2002-07-01). "Guanine nucleotide exchange factors for Rho GTPases: turning on the switch" (in en). Genes & Development 16 (13): 1587–1609. doi:10.1101/gad.1003302. ISSN 0890-9369. PMID 12101119. http://genesdev.cshlp.org/content/16/13/1587.
 |

