Chemistry:Iron(II) tetrafluoroborate
From HandWiki
| |
| Names | |
|---|---|
| Systematic IUPAC name
iron(2+);ditetrafluoroborate | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
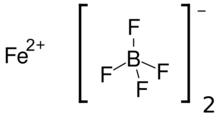
| Fe(BF4)2 | |
| Molar mass | 229.46 g/mol (anhydrous) 337.55 g/mol (hexahydrate) |
| Appearance | Light green crystals (hexahydrate) |
| Soluble | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H312, H314, H332 | |
| P260, P261, P264, P270, P271, P280, P301+312, P301+330+331, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P310, P312, P321, P322, P330, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Iron(II) tetrafluoroborate or ferrous tetrafluoroborate is an inorganic chemical with chemical formula Fe(BF4)2. Both the anhydrous form and a hexahydrate are known. The hexahydrate and aqueous solutions are green. Tetrafluoroborate is generally a weakly coordinating anion, so iron(II) tetrafluoroborate is used as the starting material for forming various other iron(II) coordination complexes.
For example, a complex composed of iron(II) tetrafluoroborate and the ligand tris[2-(diphenylphosphino)-ethyl]phosphine catalyzes the transfer hydrogenation of various aldehydes to give the corresponding primary alcohols, using formic acid as hydrogen donor.[1]
References
- ↑ Gerrit Wienhöfer; Felix A.Westerhaus; Kathrin Junge; Matthias Beller (2013). "Fast and selective iron-catalyzed transfer hydrogenations of aldehydes". Journal of Organometallic Chemistry 744: 156–159. doi:10.1016/j.jorganchem.2013.06.010.
 |

