Chemistry:Canavanine
| |
| Names | |
|---|---|
| Preferred IUPAC name
Canavanine | |
| Systematic IUPAC name
(2S)-2-amino-4-{[(diaminomethylidene)amino]oxy}butanoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
| MeSH | Canavanine |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H12N4O3 | |
| Molar mass | 176.176 g·mol−1 |
| Density | 1.61 g·cm−3 (predicted) |
| Melting point | 184 °C (363 °F; 457 K) |
| Boiling point | 366 °C (691 °F; 639 K) |
| soluble | |
| Solubility | insoluble in alcohol, ether, benzene |
| log P | -0.91 (predicted) |
| Vapor pressure | 1.61 μPa (predicted) |
| Acidity (pKa) | 2.35 (carboxylic acid), 7.01 (oxoguanidinium), 9.22 (ammonium) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H312, H332 | |
| Flash point | 214.6 °C (418.3 °F; 487.8 K) (predicted) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
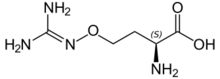
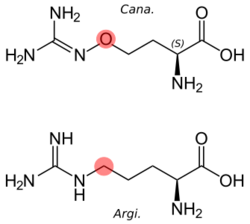
L-(+)-(S)-Canavanine is a non-proteinogenic amino acid found in certain leguminous plants. It is structurally related to the proteinogenic α-amino acid L-arginine, the sole difference being the replacement of a methylene bridge (-CH2- unit) in arginine with an oxa group (i.e., an oxygen atom) in canavanine. Canavanine is accumulated primarily in the seeds of the organisms which produce it, where it serves both as a highly deleterious defensive compound against herbivores (due to cells mistaking it for arginine) and a vital source of nitrogen for the growing embryo.[citation needed] The related L-canaline is similar to ornithine.
Toxicity
The mechanism of canavanine's toxicity is that organisms that consume it typically mistakenly incorporate it into their own proteins in place of L-arginine, thereby producing structurally aberrant proteins that may not function properly. Cleavage by arginase also produces canaline, a potent insecticide.
The toxicity of canavanine may be enhanced under conditions of protein starvation,[1] and canavanine toxicity, resulting from consumption of Hedysarum alpinum seeds with a concentration of 1.2% canavanine weight/weight, has been implicated in the death of a malnourished Christopher McCandless.[2] (McCandless was the subject of Jon Krakauer's book (and subsequent movie) Into the Wild).
In mammals
NZB/W F1, NZB, and DBA/2 mice fed L-canavanine develop a syndrome similar to systemic lupus erythematosus,[1] while BALB/c mice fed a steady diet of protein containing 1% canavanine showed no change in lifespan.[3]
Alfalfa seeds and sprouts contain L-canavanine. The L-canavanine in alfalfa has been linked to lupus-like symptoms in primates, including humans, and other auto-immune diseases. Often stopping consumption reverses the problem.[4][5][6]
Tolerance
Some specialized herbivores tolerate L-canavanine either because they metabolize it efficiently (cf. L-canaline) or avoid its incorporation into their own nascent proteins.
By metabolic detoxification
Herbivores may be able to metabolize canavanine efficiently. The beetle Caryedes brasiliensis is able to break canavanine down to canaline, then further detoxifies canaline by reductive deamination to form homoserine and ammonia. As a result, the beetle not only tolerates the chemical, but uses it as a source of nitrogen to synthesize its other amino acids to allow it to develop.[7]
By selectivity
An example of this ability can be found in the larvae of the tobacco budworm Heliothis virescens, which can tolerate massive amounts of dietary canavanine. These larvae fastidiously avoid incorporation of L-canavanine into their nascent proteins (presumably by virtue of highly discriminatory arginine—tRNA ligase, the enzyme responsible for the first step in the incorporation of arginine into proteins). In contrast, larvae of the tobacco hornworm Manduca sexta can only tolerate tiny amounts (1.0 microgram per kilogram of fresh body weight) of dietary canavanine because their arginine-tRNA ligase has little, if any, discriminatory capacity. No one has examined experimentally the arginine-tRNA synthetase of these organisms. But comparative studies of the incorporation of radiolabeled L-arginine and L-canavanine have shown that in Manduca sexta, the ratio of incorporation is about 3 to 1.[8]
Dioclea megacarpa seeds contain high levels of canavanine. The beetle Caryedes brasiliensis is able to tolerate this however as it has the most highly discriminatory arginine-tRNA ligase known (as of 1982). In this insect, the level of radiolabeled L-canavanine incorporated into newly synthesized proteins is barely measurable. Moreover, this beetle uses canavanine as a nitrogen source (see above).[9]
See also
References
- ↑ 1.0 1.1 Akaogi, Jun; Barker, Tolga; Kuroda, Yoshiki; Nacionales, Dina C.; Yamasaki, Yoshioki; Stevens, Bruce R.; Reeves, Westley H.; Satoh, Minoru (2006). "Role of non-protein amino acid l-canavanine in autoimmunity". Autoimmunity Reviews 5 (6): 429–35. doi:10.1016/j.autrev.2005.12.004. PMID 16890899.
- ↑ Krakauer, J., et al. (2015). "Presence of l-canavanine in Hedysarum alpinum seeds and its potential role in the death of Christopher McCandless." Wilderness & Environmental Medicine. doi:10.1016/j.wem.2014.08.014
- ↑ Brown, Dan L (2005). "Canavanine-induced longevity in mice may require diets with greater than 15.7% protein". Nutrition & Metabolism 2 (1): 7. doi:10.1186/1743-7075-2-7. PMID 15733319.
- ↑ Montanaro, A; Bardana Jr, E. J. (1991). "Dietary amino acid-induced systemic lupus erythematosus". Rheumatic Disease Clinics of North America 17 (2): 323–32. doi:10.1016/S0889-857X(21)00573-1. PMID 1862241.
- ↑ Herbert, V; Kasdan, T. S. (1994). "Alfalfa, vitamin E, and autoimmune disorders". The American Journal of Clinical Nutrition 60 (4): 639–40. doi:10.1093/ajcn/60.4.639. PMID 8092103.[unreliable medical source?]
- ↑ http://vegpeace.org/rawfoodtoxins.html[full citation needed][yes|permanent dead link|dead link}}][unreliable medical source?]
- ↑ Rosenthal, Gerald A.; Dahlman, D. L.; Janzen, Daniel H. (3 November 1978). "L-Canaline Detoxification: A Seed Predator's Biochemical Mechanism". Science 202 (4367): 528–529. doi:10.1126/science.202.4367.528.
- ↑ Rosenthal, G. A.; Dahlman, D. L. (1986). "L-Canavanine and protein synthesis in the tobacco hornworm Manduca sexta". Proceedings of the National Academy of Sciences 83 (1): 14–8. doi:10.1073/pnas.83.1.14. PMID 3455753. Bibcode: 1986PNAS...83...14R.
- ↑ Rosenthal, G. A.; Hughes, C. G.; Janzen, D. H. (1982). "L-Canavanine, a Dietary Nitrogen Source for the Seed Predator Caryedes brasiliensis (Bruchidae)". Science 217 (4557): 353–5. doi:10.1126/science.217.4557.353. PMID 17791516. Bibcode: 1982Sci...217..353R.
Bibliography
- Rosenthal, Gerald A. (1986). "Biochemical insight into insecticidal properties ofl-Canavanine, a higher plant protective allelochemical". Journal of Chemical Ecology 12 (5): 1145–56. doi:10.1007/BF01639001. PMID 24307052.
- Rosenthal, G. A.; Berge, M. A.; Bleiler, J. A.; Rudd, T. P. (1987). "Aberrant, canavanyl protein formation and the ability to tolerate or utilize L-canavanine". Experientia 43 (5): 558–61. doi:10.1007/BF02143585. PMID 3582574.
- Boyar, A.; Marsh, R. E. (1982). "L-Canavanine, a paradigm for the structures of substituted guanidines". Journal of the American Chemical Society 104 (7): 1995–1998. doi:10.1021/ja00371a033.
- "Distribution of canavanine in the plant kingdom". Phytochemistry 6 (6): 863–866. 1967. doi:10.1016/S0031-9422(00)86033-1. Bibcode: 1967PChem...6..863T. and in particularly large amounts in Canavalia gladiata (sword bean).
- "Canavanine content in sword beans (Canavalia gladiata): analysis and effect of processing". Food and Chemical Toxicology 45 (5): 797–803. May 2007. doi:10.1016/j.fct.2006.10.030. PMID 17187914.
.........
 |
