Chemistry:Citrulline
| |
| |
| Names | |
|---|---|
| IUPAC name
2-Amino-5-(carbamoylamino)pentanoic acid[1]
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 3DMet | |
| 1725417, 1725415 D, 1725416 L | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| 774677 L | |
| KEGG | |
| MeSH | Citrulline |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C6H13N3O3 | |
| Molar mass | 175.188 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| log P | −1.373 |
| Acidity (pKa) | 2.508 |
| Basicity (pKb) | 11.489 |
| Thermochemistry | |
Heat capacity (C)
|
232.80 J K−1 mol−1 |
Std molar
entropy (S |
254.4 J K−1 mol−1 |
| Related compounds | |
Related alkanoic acids
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
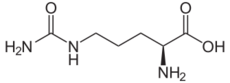
The organic compound citrulline is an α-amino acid.[2] Its name is derived from citrullus, the Latin word for watermelon. Although named and described by gastroenterologists since the late 19th century, it was first isolated from watermelon in 1914 by Japan ese researchers Yotaro Koga and Ryo Odake[3][note 1] and further codified by Mitsunori Wada of Tokyo Imperial University in 1930.[4] It has the formula H2NC(O)NH(CH2)3CH(NH2)CO2H. It is a key intermediate in the urea cycle, the pathway by which mammals excrete ammonia by converting it into urea. Citrulline is also produced as a byproduct of the enzymatic production of nitric oxide from the amino acid arginine, catalyzed by nitric oxide synthase.[5]
Biosynthesis
Citrulline can be derived from:
- from arginine via nitric oxide synthase, as a byproduct of the production of nitric oxide for signaling purposes
- from ornithine through the breakdown of proline or glutamine/glutamate
- from asymmetric dimethylarginine via DDAH
Citrulline is made from ornithine and carbamoyl phosphate in one of the central reactions in the urea cycle. It is also produced from arginine as a byproduct of the reaction catalyzed by NOS family (NOS; EC 1.14.13.39).[6] It is also prevalent in trichohyalin at the inner root sheath and medulla of hair follicles, where it is synthesized from arginine.[7] Arginine is first oxidized into N-hydroxyl-arginine, which is then further oxidized to citrulline concomitant with release of nitric oxide.
Citrulline is also made by enterocytes of the small intestine.[2][8]
Function
Citrulline is a metabolic intermediate within the urea cycle, which is the pathway by which mammals excrete ammonia by converting it into urea. Citrulline is also produced as a byproduct of the enzymatic production of nitric oxide from the amino acid arginine, catalyzed by nitric oxide synthase. In the yeast species Saccharomyces cerevisiae, citrulline is a metabolic intermediate in the latter, cytosolic half of the arginine biosynthesis pathway.[9]
Several proteins contain citrulline as a result of a post-translational modification. These citrulline residues are generated by a family of enzymes called peptidylarginine deiminases (PADs), which convert arginine into citrulline in a process called citrullination or deimination with the help of calcium ions. Proteins that normally contain citrulline residues include myelin basic protein (MBP), filaggrin, and several histone proteins, whereas other proteins, such as fibrin and vimentin are susceptible to citrullination during cell death and tissue inflammation.
Circulating citrulline concentration is a biomarker of intestinal functionality.[10][11]
See also
Notes
- ↑ Early references spell Ryo Odake's name as Ryo Othake.
References
- ↑ "Citrulline - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=833&loc=ec_rcs#x291.
- ↑ 2.0 2.1 Banerjee, Aryamitra (2014-01-01), Gupta, Ramesh C., ed., "Chapter 15 - Gastrointestinal toxicity biomarkers" (in en), Biomarkers in Toxicology (Boston: Academic Press): pp. 269–277, doi:10.1016/b978-0-12-404630-6.00015-4, ISBN 978-0-12-404630-6, http://www.sciencedirect.com/science/article/pii/B9780124046306000154, retrieved 2020-11-10
- ↑ Fragkos, Konstantinos C.; Forbes, Alastair (September 2011). "Was citrulline first a laxative substance? The truth about modern citrulline and its isolation". Nihon Ishigaku Zasshi. [Journal of Japanese History of Medicine] 57 (3): 275–292. ISSN 0549-3323. PMID 22397107. http://discovery.ucl.ac.uk/1317394/1/1317394.pdf.
- ↑ Fearon, William Robert (1939). "The Carbamido Diacetyl Reaction: A Test For Citrulline". Biochemical Journal 33 (6): 902–907. doi:10.1042/bj0330902. PMID 16746990.
- ↑ "Nos2 - Nitric Oxide Synthase". Uniprot Consortium. https://www.uniprot.org/uniprot/P29477.
- ↑ Lehninger principles of biochemistry (3rd ed.). New York: Worth Publishers. 2000. p. 449. ISBN 978-1-57259-153-0. https://archive.org/details/lehningerprincip01lehn. Retrieved 13 March 2020.
- ↑ Rogers, G. E.; Rothnagel, J. A. (1983). "A sensitive assay for the enzyme activity in hair follicles and epidermis that catalyses the peptidyl-arginine-citrulline post-translational modification". Current Problems in Dermatology 11: 171–184. doi:10.1159/000408673. ISBN 978-3-8055-3752-0. PMID 6653155.
- ↑ DeLegge, Mark H. (2019-01-01), Corrigan, Mandy L.; Roberts, Kristen; Steiger, Ezra, eds., "Chapter 7 - Enteral Access and Enteral Nutrition in Patients With Short Bowel Syndrome" (in en), Adult Short Bowel Syndrome (Academic Press): pp. 81–96, doi:10.1016/b978-0-12-814330-8.00007-x, ISBN 978-0-12-814330-8, http://www.sciencedirect.com/science/article/pii/B978012814330800007X, retrieved 2020-11-10
- ↑ "Saccharomyces cerevisiae citrulline biosynthesis". https://pathway.yeastgenome.org/YEAST/new-image?object=CITRUL-BIO2-PWY.
- ↑ Fragkos, Konstantinos C.; Forbes, Alastair (2017-10-12). "Citrulline as a marker of intestinal function and absorption in clinical settings: A systematic review and meta-analysis" (in en). United European Gastroenterology Journal 6 (2): 181–191. doi:10.1177/2050640617737632. PMID 29511548.
- ↑ Crenn, P. (2000). "Post-absorptive plasma citrulline concentration is a marker of intestinal failure in short bowel syndrome patients". Gastroenterology 119 (6): 1496–505. doi:10.1053/gast.2000.20227. PMID 11113071.
 |
