Chemistry:Hypusine
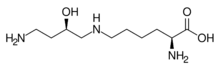
| |
| Names | |
|---|---|
| IUPAC name
N6-[(2R)-4-amino-2-hydroxybutyl]-L-lysine
| |
| Systematic IUPAC name
(2S)-2-Amino-6-{[(2R)-4-amino-2-hydroxybutyl]amino}hexanoic acid | |
| Other names
N6-(4-Amino-2-hydroxybutyl)lysine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | Hypusine |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H23N3O3 | |
| Molar mass | 233.312 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hypusine is an uncommon amino acid found in all eukaryotes and in some archaea, but not in bacteria. The only known proteins containing the hypusine residue is eukaryotic translation initiation factor 5A (eIF-5A) and a similar protein found in archaea.[1] In humans, two isoforms of eIF-5A have been described: eIF5A-1 and eIF5A-2. They are encoded by two distinct genes EIF5A and EIF5A2. The protein is involved in protein biosynthesis and promotes the formation of the first peptide bond. The region surrounding the hypusine residue is highly conserved and is essential to the function of eIF5A.[2] Thus, hypusine and eIF-5A appear to be vital for the viability and proliferation of eukaryotic cells.
Hypusine is formed in eIF-5A by post-translational modification of one of the lysyl residues. Two reactions and two enzymes are involved:
- 1. Deoxyhypusine synthase catalyzes the cleavage of the polyamine spermidine and transfer of its 4-aminobutyl moiety to the ε-amino group of one specific lysine residue of the eIF-5A precursor to form deoxyhypusine and 1,3-diaminopropane.
- 2. Deoxyhypusine hydroxylase mediates the formation of hypusine by addition of a hydroxyl group to the deoxyhypusine residue.
An excess of hypusine was found in the urine of children and patients with familial hyperlysinemia.
Hypusine was first isolated from bovine brain by Japanese scientists Shiba et al. in 1971.[3] The name hypusine indicates that the molecule comprises moieties of hydroxyputrescine and lysine.
See also
- n-Butylamine, related to 4-aminobutyl group of deoxyhypusine
- Putrescine
- Diphthamide, another translation-related uncommon amino acid
- EEF2, eukaryotic elongation factor 2, utilizing diphthamide
References
- ↑ Park MH (2006). "The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A)". Journal of Biochemistry 139 (2): 161–169. doi:10.1093/jb/mvj034. PMID 16452303.
- ↑ "Mutational analyses of human eIF5A-1 -- Identification of amino acid residues critical for eIF5A activity and hypusine modification". FEBS Journal 275 (1): 44–58. 2008. doi:10.1111/j.1742-4658.2007.06172.x. PMID 18067580.
- ↑ "Hypusine, a new amino acid occurring in bovine brain. Isolation and structural determination". Biochimica et Biophysica Acta 244 (3): 523–531. 1971. doi:10.1016/0304-4165(71)90069-9. PMID 4334286.
 |
