Chemistry:Magnesium chlorate
| |
| Names | |
|---|---|
| IUPAC name
Magnesium dichlorate hexahydrate
| |
| Systematic IUPAC name
Magnesium dichlorate | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2723 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Mg(ClO3)2 | |
| Molar mass | 191.20 g/mol |
| Appearance | White crystalline solid |
| Density | 1.747 g/cm3 (hexahydrate)[1] |
| Melting point | 35 °C (95 °F; 308 K)[2] |
| Boiling point | 120 °C (248 °F; 393 K)[2] (decomposition) |
| 114 g/100 ml (0 °C) 123 g/100 ml (10 °C) 135 g/100 ml (20 °C) 155 g/100 ml (30 °C) 178 g/100 ml (50 °C) 242 g/100 ml (60 °C) 268 g/100 ml (100 °C)[2] | |
| Solubility in acetone | Soluble |
| Structure[1] | |
| Monoclinic | |
| P21/c | |
a = 6.39 Å, b = 6.51 Å, c = 13.90 Å α = 90°, β = 100.3°, γ = 90°
| |
Lattice volume (V)
|
590.1 Å3 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H332 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
6348 mg/kg (rat, oral) |
| Related compounds | |
Other cations
|
Calcium chlorate Strontium chlorate Barium chlorate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Magnesium chlorate refers to inorganic compounds with the chemical formula Mg(ClO3)2(H2O)x. The anhydrous (x = 0), dihydrate (x = 2), and hexahydrate (x = 6) are known. These are thermally labile white solids. The hexahydrate has been identified on the Martian surface.[3]
Production
Samples of magnesium chlorate were first claimed in 1920 as the result of treating magnesium oxide with chlorine. A more modern method involves electrolysis of magnesium chloride.[4] The magnesium chlorate can be purified by exploiting its solubility in acetone.[4]
Properties
The hexahydrate Mg(ClO3)2·6H2O decomposes to the tetrahydrate at 35 °C. At 65 °C, it dehydrates to the dihydrate, then at 80 °C forms a basic salt. If further heated to 120 °C it decomposes to water, oxygen, chlorine, and magnesium oxide.[2]
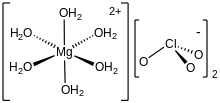
As confirmed by X-ray crystallography, the di- and hexahydrates feature octahedral Mg2+ centers. The other ligands are water, exclusively in the hexahydrate. In the dihydrate, chlorate is also coordinated and functions as a bridging ligand.[1]
Uses
Magnesium(II) chlorate is used as a powerful desiccant and a defoliant for cotton, potato, and rice. It is also found as a lubricant in eye drops as an inactive ingredient.[5]
Hazards
Magnesium chlorate is an oxidizer and can in principle form explosive mixtures.
References
- ↑ 1.0 1.1 1.2 Kossev, K; Tsvetanova, L.; Dimowa, L.; Nikolova, R.; Shivachev, B. (2013). "Synthesis and Crystal Structure of Magnesium Chlorate Dihydrate and Magnesium Chlorate Hexahydrate". Bulgarian Chemical Communications 45: 543–548.
- ↑ 2.0 2.1 2.2 2.3 Joseph William Mellor (1922). Supplement to Mellor's Comprehensive Treatise on Inorganic and Theoretical Chemistry: suppl. 3. K, Rb, Cs, Fr. Longmans, Green and Company.
- ↑ Ojha, Lujendra; Wilhelm, Mary Beth; Murchie, Scott L.; McEwen, Alfred S.; Wray, James J.; Hanley, Jennifer; Massé, Marion; Chojnacki, Matt (2015). "Spectral evidence for hydrated salts in recurring slope lineae on Mars". Nature Geoscience 8 (11): 829–832. doi:10.1038/ngeo2546. Bibcode: 2015NatGe...8..829O.
- ↑ 4.0 4.1 Herbert Maxim (1948) (in English). The electrolytic production of magnesium chlorate and perchlorate. the Department of Chemical Engineering: University of Southern California.
- ↑ "MAGNESIUM CHLORATE" (in English). U.S. Department of Health & Human Services. https://drugs.ncats.io/substance/M536P01U3N#general.
 |

