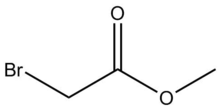
Chemistry:Methyl 2-bromoacetate
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl bromoacetate | |
| Other names
Bromoacetic acid methyl ester, Methyl α-bromoacetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C3H5BrO2 | |
| Molar mass | 152.975 g·mol−1 |
| Density | 1.6±0.1 g/cm3[1] |
| Boiling point | 154 °C (309 °F; 427 K) |
| Solubility | Soluble in water |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | WARNING |
| H301, H311, H314, H335 | |
| P260, P261, P264, P270, P271, P280, P301, P310, P330, P331, P302, P352, P303, P361, P353, P304, P340, P305, P351, P338, P312, P321, P322, P363, P403 | |
| Flash point | 63 °C (145 °F; 336 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methyl 2-bromoacetate (methyl bromoactate) is a chemical compound with the molecular formula C3H5BrO2.
Properties
Methyl 2-bromoacetate is colorless or straw-colored liquid. The smell is sharp and penetrating. It is soluble in water and also has a higher density than water.[2] It is incompatible with acids, bases, oxidizing agents, and reducing agents.[3]
Application
Methyl bromoacetate is an alkylating agent. It has been used to alkylate phenol and amino groups.[4][5] Moreover, it can be used to make vitamins and pharmaceutical drugs. It is commonly used as a reagent in chemical modification of histidine.[2] In addition, methyl bromoacetate also use in synthesize of coumarins and cis-cyclopropane. It reacts with conjugated base and produce alkylated carbene complexes.[3]
Safety
Methyl bromoacetate can be toxic by ingestion and inhalation. It can also irritate the skin and eyes.[2]
See also
References
- ↑ "CSID:54945". ChemSpider. http://www.chemspider.com/Chemical-Structure.54945.html.
- ↑ 2.0 2.1 2.2 "Methyl Bromoacetate - Compound Summary for CID 60984". PubChem Compound Database. USA: National Center for Biotechnology Information. Identification. https://pubchem.ncbi.nlm.nih.gov/compound/Methyl_bromoacetate#section=Top.
- ↑ 3.0 3.1 "A10605 Methyl bromoacetate, 98+%". Alfa Aesar. https://www.alfa.com/en/catalog/A10605/.
- ↑ Piątek, Piotr; Jurczak, Janusz (25 September 2002). "A selective colorimetric anion sensor based on an amide group containing macrocycle†". The Royal Society of Chemistry.
- ↑ Raju, B.; Murphy, E.; Levy, L.A.; Hall, R.D.; London, R.E. (1 March 1989). "A fluorescent indicator for measuring cytosolic free magnesium". The American Journal of Physiology 256 (3 Pt 1): C540-8. doi:10.1152/ajpcell.1989.256.3.C540. PMID 2923192.
Extra reading
- Raju, B.; Murphy, E.; Levy, L. A.; Hall, R. D.; London, R. E. (1 March 1989). "A fluorescent indicator for measuring cytosolic free magnesium". American Journal of Physiology. Cell Physiology 256 (3): C540–C548. doi:10.1152/ajpcell.1989.256.3.C540. PMID 2923192.
- Upper, Christen D.; West, Charles A. (July 1967). "Biosynthesis of Gibberellins". Journal of Biological Chemistry 242 (14): 3285–3292. doi:10.1016/S0021-9258(18)95908-9.
- Davis, Franklin A.; Zhou, Ping; Reddy, G. Venkat (June 1994). "Asymmetric Synthesis and Reactions of cis-N-(p-Toluenesulfinyl)aziridine-2-carboxylic Acids". The Journal of Organic Chemistry 59 (12): 3243–3245. doi:10.1021/jo00091a001.
- Henderson, Jaclyn L.; Edwards, Andrew S.; Greaney, Michael F. (June 2006). "Three-Component Coupling of Benzyne: Domino Intermolecular Carbopalladation". Journal of the American Chemical Society 128 (23): 7426–7427. doi:10.1021/ja0615526. PMID 16756281.
- Hannick, Steven M.; Kishi, Yoshito (October 1983). "An improved procedure for the Blaise reaction: a short, practical route to the key intermediates of the saxitoxin synthesis". The Journal of Organic Chemistry 48 (21): 3833–3835. doi:10.1021/jo00169a053.
 |

