Chemistry:NCS-382
From HandWiki
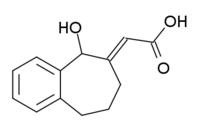
| |
| Names | |
|---|---|
| IUPAC name
(2E)-(5-hydroxy-5,7,8,9-tetrahydro-6H-benzo[a][7]annulen-6-ylidene ethanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | NCS-382 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H14O3 | |
| Molar mass | 218.248 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
NCS-382 is a moderately selective antagonist for the GHB receptor.[1][2] It blocks the effects of GHB in animals and has both anti-sedative and anticonvulsant effects.[3][4][5] It has been proposed as a treatment for GHB overdose in humans as well as the genetic metabolic disorder succinic semialdehyde dehydrogenase deficiency (SSADHD), but has never been developed for clinical use.[6]
References
- ↑ "A review of pharmacology of NCS-382, a putative antagonist of gamma-hydroxybutyric acid (GHB) receptor". CNS Drug Reviews 10 (3): 243–260. 2004. doi:10.1111/j.1527-3458.2004.tb00025.x. PMID 15492774.
- ↑ "Characterization and pharmacology of the GHB receptor". Annals of the New York Academy of Sciences 1139 (1): 374–385. October 2008. doi:10.1196/annals.1432.048. PMID 18991884. Bibcode: 2008NYASA1139..374T.
- ↑ "A specific gamma-hydroxybutyrate receptor ligand possesses both antagonistic and anticonvulsant properties". Journal of Pharmacology and Experimental Therapeutics 255 (2): 657–63. Nov 1990. PMID 2173754. http://jpet.aspetjournals.org/content/255/2/657.abstract.
- ↑ "Anti-sedative and anti-cataleptic properties of NCS-382, a gamma-hydroxybutyrate receptor antagonist". European Journal of Pharmacology 203 (3): 393–7. Oct 1991. doi:10.1016/0014-2999(91)90896-X. PMID 1773824.
- ↑ "Blockade of the discriminative stimulus effects of gamma-hydroxybutyric acid (GHB) by the GHB receptor antagonist NCS-382". Physiology & Behavior 58 (3): 587–590. Sep 1995. doi:10.1016/0031-9384(95)00086-X. PMID 8587968.
- ↑ "Therapeutic intervention in mice deficient for succinate semialdehyde dehydrogenase (gamma-hydroxybutyric aciduria)". Journal of Pharmacology and Experimental Therapeutics 302 (1): 180–187. Jul 2002. doi:10.1124/jpet.302.1.180. PMID 12065715.
 |
