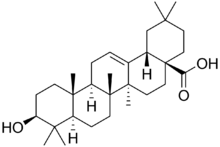
Chemistry:Oleanolic acid
| |
| Names | |
|---|---|
| IUPAC name
3β-Hydroxyolean-12-en-28-oic acid
| |
| Systematic IUPAC name
(4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-10-Hydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylic acid | |
| Other names
Oleanic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C30H48O3 | |
| Molar mass | 456.711 g·mol−1 |
| Appearance | White |
| Melting point | > 300 °C (572 °F; 573 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid related to betulinic acid. It is widely distributed in food and plants where it exists as a free acid or as an aglycone of triterpenoid saponins.[2]
Natural occurrence
Oleanolic acid can be found in olive oil, Phytolacca americana (American pokeweed), and Syzygium spp, garlic, etc. It was first studied and isolated from several plants, including Olea europaea[3] (leaves, fruit), Rosa woodsii (leaves), Prosopis glandulosa (leaves and twigs), Phoradendron juniperinum (whole plant), Syzygium claviflorum (leaves), Hyptis capitata (whole plant), Mirabilis jalapa[4] and Ternstroemia gymnanthera (aerial part). Other Syzygium species including java apple (Syzygium samarangense) and rose apples contain it, as does Ocimum tenuiflorum (holy basil).
Biosynthesis of oleanolic acids
Oleanolic acid biosynthesis starts with mevalonate to create squalene. Squalene monooxygenase in the next step oxidases the squalene and forms an epoxide resulting in 2,3-oxidosqualene.[5] Beta-amyrin synthase creates beta-amyrin by a ring formation cascade.[5][6] After the formation of beta amyrin, CYP716AATR2, also known as a cytochrome p450 enzyme, oxidizes carbon 28 turning it into alcohol.[6] CYP716AATR2 converts the alcohol to aldehyde and finally to a carboxylic acid forming oleanolic acid.[6]

Pharmacological research
Oleanolic acid is relatively non-toxic, hepatoprotective, and exhibits antitumor and antiviral properties.[7] Oleanolic acid was found to exhibit weak anti-HIV[8] and weak anti-HCV activities in vitro, but more potent synthetic analogs are being investigated as potential drugs.[9]
An extremely potent synthetic triterpenoid analog of oleanolic acid was found in 2005, that is a powerful inhibitor of cellular inflammatory processes. They work by the induction by IFN-γ of inducible nitric oxide synthase (iNOS) and of cyclooxygenase 2 in mouse macrophages. They are extremely potent inducers of the phase 2 response (e.g., elevation of NADH-quinone oxidoreductase and heme oxygenase 1), which is a major protector of cells against oxidative and electrophile stress.[10]
A 2002 study in Wistar rats found that oleanolic acid reduced sperm quality and motility, causing infertility. After withdrawing exposure, male rats regained fertility and successfully impregnated female rats.[11] Oleanolic acid is also used as standard for comparison of hyaluronidase, elastase and matrix-metalloproteinase-1 inhibition of other substances in primary research (similar to diclofenac sodium for comparison of analgesic activity).[12][13]
Oleanolic acid activates telomerase in peripheral blood mononuclear cells (PBMCs) 5.9-fold, more than any other compounded tested, with the exception of Centella asiatica (8.8-fold).[14] Less telomerase activation is seen for Astragalus extract 4.3-fold, TA-65 2.2-fold, and maslinic acid 2-fold.[14]
See also
- Ursolic acid
- Betulinic acid
- Moronic acid
- Momordin (saponin), a glycoside of oleanolic acid
- List of phytochemicals in food
References
- ↑ "Oleanolic acid". Merck. https://www.sigmaaldrich.com/catalog/product/aldrich/o5504?lang=en®ion=GB.
- ↑ "Oleanolic acid". Phytochemistry 77: 10–15. May 2012. doi:10.1016/j.phytochem.2011.12.022. PMID 22377690.
- ↑ "Oleanolic acid (HMDB0002364)". Canadian Institutes of Health Research. http://www.hmdb.ca/metabolites/HMDB0002364.
- ↑ "Constituents of Mirabilis jalapa". Fitoterapia 61 (5): 471. 1990. http://www.cabdirect.org/abstracts/19910302341.html.)
- ↑ 5.0 5.1 "CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis". Plant & Cell Physiology 52 (12): 2050–2061. December 2011. doi:10.1093/pcp/pcr146. PMID 22039103.
- ↑ 6.0 6.1 6.2 "A systematic comparison of triterpenoid biosynthetic enzymes for the production of oleanolic acid in Saccharomyces cerevisiae". PLOS ONE 15 (5). 2020-05-01. doi:10.1371/journal.pone.0231980. PMID 32357188. Bibcode: 2020PLoSO..1531980D.
- ↑ "Pharmacology of oleanolic acid and ursolic acid". Journal of Ethnopharmacology 49 (2): 57–68. December 1995. doi:10.1016/0378-8741(95)90032-2. PMID 8847885.
- ↑ "In vitro anti-HIV activity of oleanolic acid on infected human mononuclear cells". Planta Medica 68 (2): 111–114. February 2002. doi:10.1055/s-2002-20256. PMID 11859458.
- ↑ "Development of oleanane-type triterpenes as a new class of HCV entry inhibitors". Journal of Medicinal Chemistry 56 (11): 4300–4319. June 2013. doi:10.1021/jm301910a. PMID 23662817.
- ↑ "Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress". Proceedings of the National Academy of Sciences of the United States of America 102 (12): 4584–4589. March 2005. doi:10.1073/pnas.0500815102. PMID 15767573. Bibcode: 2005PNAS..102.4584D.
- ↑ "The effect of oleanolic acid on sperm motion characteristics and fertility of male Wistar rats". Laboratory Animals 36 (4): 432–437. October 2002. doi:10.1258/002367702320389107. PMID 12396287.
- ↑ "Standardized Clitoria ternatea leaf extract as hyaluronidase, elastase and matrix-metalloproteinase-1 inhibitor". Indian Journal of Pharmacology 44 (5): 584–587. 2012. doi:10.4103/0253-7613.100381. PMID 23112418.
- ↑ "Matrix metalloproteinase, hyaluronidase and elastase inhibitory potential of standardized extract of Centella asiatica". Pharmaceutical Biology 51 (9): 1182–1187. September 2013. doi:10.3109/13880209.2013.782505. PMID 23763301.
- ↑ 14.0 14.1 "Discovery of potent telomerase activators: Unfolding new therapeutic and anti-aging perspectives". Molecular Medicine Reports 20 (4): 3701-3708. 2019. doi:10.3892/mmr.2019.10614. PMID 31485647.
 |
