Chemistry:Potassium hydrosulfide
| |
| Names | |
|---|---|
| IUPAC name
Potassium hydrosulfide
| |
| Other names
Potassium bisulfide, Potassium sulfhydrate, potassium hydrogen sulfide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| KSH | |
| Molar mass | 72.171 g/mol |
| Appearance | white solid |
| Density | 1.68–1.70 g/cm3 |
| Melting point | 455 °C (851 °F; 728 K) |
| good | |
| Hazards | |
| Main hazards | Flammable solid, stench, releases hydrogen sulfide |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Potassium hydroxide |
Other cations
|
Sodium hydrosulfide |
Related compounds
|
potassium sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium hydrosulfide is the inorganic compound with the formula KSH. This colourless salt consists of the cation K+
and the bisulfide anion [SH]−
. It is the product of the half-neutralization of hydrogen sulfide with potassium hydroxide. The compound is used in the synthesis of some organosulfur compounds.[1] Aqueous solutions of potassium sulfide consist of a mixture of potassium hydrosulfide and potassium hydroxide.
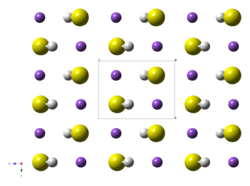
The structure of the potassium hydrosulfide resembles that for potassium chloride. Their structure is however complicated by the non-spherical symmetry of the SH−
anions, but these tumble rapidly in the solid.[2]
Addition of sulfur gives dipotassium pentasulfide.
Synthesis
It is prepared by neutralizing aqueous KOH with H
2S.[3][4]
References
- ↑ Dittmer, Donald C. (2001). Paquette, L.. ed. Encyclopedia of Reagents for Organic Synthesis. J. Wiley & Sons, New York. doi:10.1002/047084289X.rp227. ISBN 0471936235.
- ↑ Haarmann, F; Jacobs, H.; Roessler, E.; Senker, J. (2002). "Dynamics of Anions and Cations in Hydrogensulfides of Alkali Metals (NaHS, KHS, RbHS): A Proton Nuclear Magnetic Resonance Study". Journal of Chemical Physics 117 (3): 1269–1276. doi:10.1063/1.1483860. Bibcode: 2002JChPh.117.1269H.
- ↑ Kurzer, F.; Lawson, A. (1962). "Thiobenzoylthioglycolic Acid". Organic Syntheses 42: 100. doi:10.15227/orgsyn.042.0100. http://www.orgsyn.org/demo.aspx?prep=CV5P1046.
- ↑ Robert L. Frank and James R. Blegen (1948). "Benzoyl Disulfide". Organic Syntheses 28: 16. doi:10.15227/orgsyn.028.0016.
 |


