Chemistry:Silver lactate
| |
| Names | |
|---|---|
| Other names
silver; 1-hydroxy-1-oxopropan-2-olate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
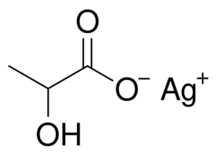
| CH3CH(OH)COOAg | |
| Molar mass | 196.93 g/mol |
| Appearance | Gray to purple powder or flakes |
| Melting point | 120–122 °C (248–252 °F; 393–395 K) |
| Boiling point | 227.6 °C (441.7 °F; 500.8 K) |
| Soluble | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P302, P352, P305, P351, P338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Silver lactate is an organic chemical compound, a salt of silver and lactic acid[1] with the formula CH3CH(OH)COOAg.[2][3]
Synthesis
Silver lactate can be made by the reaction of silver carbonate with lactic acid.
Physical properties
Silver lactate forms light gray crystals.[4]
Silver lactate is soluble in water, slightly soluble in ethanol.
Silver lactate forms a crystalline hydrate of composition CH3CH(OH)COOAg•H2O.
Silver lactate is a reagent for the precipitation of uric acid.[5]
Chemical properties
The compound reacts with triphenylphosphine gold chloride in a mixed solvent of benzene and dichloromethane to obtain colorless triphenylphosphine gold lactate.[6]
The compound reacts with a tetraphosphine ligand, dppbpda, to obtain a coordination polymer [(dppbpda)Ag4(CH3CH(OH)COO)4]n.[7]
References
- ↑ Hacker, Gerhard W.; Gu, Jiang (17 April 2002) (in en). Gold and Silver Staining: Techniques in Molecular Morphology. CRC Press. p. 62. ISBN 978-1-4200-4023-4. https://books.google.com/books?id=FSLNBQAAQBAJ&dq=silver+lactate&pg=PA62. Retrieved 18 January 2022.
- ↑ "Silver Lactate". American Elements. https://www.americanelements.com/silver-lactate-128-00-7.
- ↑ "Silver lactate". Sigma Aldrich. https://www.sigmaaldrich.com/RU/en/product/aldrich/359750.
- ↑ Hayat, M. A. (3 August 1995) (in en). Immunogold-Silver Staining: Principles, Methods, and Applications. CRC Press. p. 30. ISBN 978-0-8493-2449-9. https://books.google.com/books?id=kjk9SY0b5NcC&dq=silver+lactate+monohydrate&pg=PA30. Retrieved 18 January 2022.
- ↑ (in en) Cornell University Medical Bulletin. 1928. p. 296. https://books.google.com/books?id=YI1XAAAAMAAJ&q=silver+lactate. Retrieved 18 January 2022.
- ↑ Fackler, John P.; Khan, M. Nazrul I.; King, Christopher; Staples, Richard J.; Winpenny, Richard E. P. (1 July 1991). "Decarboxylation of (triphenylphosphine)gold(I) carboxylates". Organometallics 10 (7): 2178–2183. doi:10.1021/om00053a021. ISSN 0276-7333. https://pubs.acs.org/doi/abs/10.1021/om00053a021. Retrieved 23 January 2022.
- ↑ Zhang, Min; Feng, Meng-Yao; Yan, Jia-Jun; Li, Hai-Yan; Young, David James; Li, Hong-Xi; Ren, Zhi-Gang (21 June 2021). "New Silver(I)-P4 Coordination Polymers Strongly Adsorb Congo Red to Yield Composites with Enhanced Photocurrent Responses". European Journal of Inorganic Chemistry 2021 (23): 2262–2265. doi:10.1002/ejic.202100228. https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/ejic.202100228. Retrieved 23 January 2022.
 |

