Chemistry:Xenon dioxydifluoride
From HandWiki
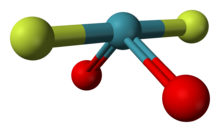
| |
| Names | |
|---|---|
| IUPAC name
Difluoro(dioxo)xenon
| |
| Other names
Xenon(VI) dioxide difluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| F2O2Xe | |
| Molar mass | 201.288 g·mol−1 |
| Melting point | 30.8 °C (87.4 °F; 304 K)[1] |
| Structure | |
| Orthorhombic [2] | |
| Disphenoidal or seesaw [Sawhorse] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Xenon dioxydifluoride is an inorganic chemical compound with the formula XeO2F2.[2][1] At room temperature it exists as a metastable solid, which decomposes slowly into xenon difluoride, but the cause of this decomposition is unknown.[1]
Preparation
Xenon dioxydifluoride is prepared by reacting xenon trioxide with xenon oxytetrafluoride.[1]
- [math]\ce{ XeO3 + XeOF4 -> 2XeO2F2 }[/math]
References
- ↑ 1.0 1.1 1.2 1.3 Claassen, Howard H.; Gasner, Earl L.; Kim, Hyunyong; Huston, J. L. (July 1968). "Vibrational Spectra and Structure of XeO 2 F 2" (in en). The Journal of Chemical Physics 49 (1): 253–257. doi:10.1063/1.1669818. ISSN 0021-9606. Bibcode: 1968JChPh..49..253C. http://aip.scitation.org/doi/10.1063/1.1669818.
- ↑ 2.0 2.1 Willett, R. D.; LaBonville, P.; Ferraro, J. R. (1975-08-15). "Normal coordinate treatment of XeO 2 F 2" (in en). The Journal of Chemical Physics 63 (4): 1474–1478. doi:10.1063/1.431510. ISSN 0021-9606. Bibcode: 1975JChPh..63.1474W. http://aip.scitation.org/doi/10.1063/1.431510.
 |

