Medicine:Apolipoprotein H
 Generic protein structure example |
β2-glycoprotein 1, also known as beta-2 glycoprotein 1 and Apolipoprotein H (Apo-H), is a 38 kDa multifunctional plasma protein that in humans is encoded by the APOH gene.[1] One of its functions is to bind cardiolipin. When bound, the structure of cardiolipin and β2-GP1 both undergo large changes in structure.[2] Within the structure of Apo-H is a stretch of positively charged amino acids (protein sequence positions 282-287), Lys-Asn-Lys-Glu-Lys-Lys, are involved in phospholipid binding (see image on right).[3]
β2-GP1 has a complex involvement in agglutination. It appears to alter adenosine diphosphate (ADP)-mediated agglutination of platelets.[4] Normally, β2-GP1 assumes an anticoagulation activity in serum (by inhibiting coagulation factors); however, changes in blood factors can result in a reversal of that activity.
Although previously referred to as apolipoprotein H, it is not present in appreciable quantities in the lipoprotein fractions, so ApoH is therefore thought to be a misnomer.[5]
Inhibitory activities
β2-GP1 appears to completely inhibit serotonin release by the platelets[6] and prevents subsequent waves of the ADP-induced aggregation. The activity of β2-GP1 appears to involve the binding of agglutinating, negatively charged compounds, and inhibits agglutination by the contact activation of the intrinsic blood coagulation pathway.[7] β2-GP1 causes a reduction of the prothrombinase binding sites on platelets and reduces the activation caused by collagen when thrombin is present at physiological serum concentrations of β2-GP1 suggesting a regulatory role of β2-GP1 in coagulation.[8]
β2-GP1 also inhibits the generation of factor Xa in the presence of platelets.[9] β2-GP1 also inhibits that activation of factor XIIa.[10]
In addition, β2-GP1 inhibits the activation of protein C blocking its activity on phosphatidylserine:phosphatidylcholine vesicles[11] however once protein C is activated, Apo-H fails to inhibit activity. Since protein C is involved in factor Va degradation Apo-H indirectly inhibits the degradation of factor Va.[12] This inhibitory activity is diminished by adding phospholipids suggesting the Apo-H inhibition of protein C is phospholipid competitive.[13] This indicates that under certain conditions Apo-H takes on procoagulation properties.
Pathology
Anti-β2-GP1 antibodies are found in both infectious and some systemic autoimmune diseases (eg. systemic lupus erythematosus (SLE)).[14] Positivity for anti-cardiolipin antibodies in diagnostic tests for autoimmune antiphospholipid syndrome requires the presence of β2-GP1in the cardiolipin extract.[15][16] Anti-β2-GP1 antibodies are strongly associated with thrombotic forms of lupus.
Sushi 2 protein domain
| Sushi_2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
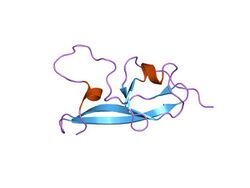 NMR structure of the fifth domain of human beta-2 glycoprotein 1 | |||||||||
| Identifiers | |||||||||
| Symbol | Sushi_2 | ||||||||
| Pfam | PF09014 | ||||||||
| InterPro | IPR015104 | ||||||||
| |||||||||
In molecular biology, the protein domain Sushi 2 is also known as the fifth protein domain of beta-2 glycoprotein 1 (β2-GP1). This protein domain is only found in eukaryotes. The first four domains found in Apolipoprotein H resemble each other, however the fifth one appears to be different.[17]
Structure
This protein domain is composed of four well-defined anti-parallel beta-strands and two short alpha-helices, as well as a long highly flexible loop.[18] Additionally, the fifth protein domain appears to resemble the other four in Apolipoprotein with the exception of three internal disulfide bonds and an extra C-terminal loop.[17]
Function
Its exact function remains to be fully elucidated; however it is known to play an important role in the binding of β2-GP1 to negatively charged compounds and subsequent capture for binding of anti-β2-GP1 antibodies.[18] Development of antibodies against β2-GP1 can lead to Antiphospholipid syndrome which often leads to pregnancy complications.[17]
References
- ↑ "APOH - Beta-2-glycoprotein 1 precursor - Homo sapiens (Human) - APOH gene & protein". https://www.uniprot.org/uniprot/P02749.
- ↑ "Interactions and molecular structure of cardiolipin and beta 2-glycoprotein 1 (beta 2-GP1)". Clin. Exp. Immunol. 102 (2): 373–8. 1995. doi:10.1111/j.1365-2249.1995.tb03792.x. PMID 7586693.
- ↑ "Site-directed mutagenesis of recombinant human beta 2-glycoprotein I identifies a cluster of lysine residues that are critical for phospholipid binding and anti-cardiolipin antibody activity". J. Immunol. 157 (8): 3744–51. 1996. doi:10.4049/jimmunol.157.8.3744. PMID 8871678.
- ↑ "Interaction of beta 2-glycoprotein-I with human blood platelets: influence upon the ADP-induced aggregation". Thromb. Haemost. 54 (2): 397–401. 1985. doi:10.1055/s-0038-1657748. PMID 4082080.
- ↑ "Beta2-glycoprotein I is incorrectly named apolipoprotein H". Journal of Thrombosis and Haemostasis 7 (1): 235–6. January 2009. doi:10.1111/j.1538-7836.2008.03223.x. PMID 19017258.
- ↑ "Beta 2-glycoprotein-I (apo-H) inhibits the release reaction of human platelets during ADP-induced aggregation". Atherosclerosis 63 (2–3): 109–14. 1987. doi:10.1016/0021-9150(87)90110-9. PMID 3827975.
- ↑ Schousboe I (1985). "beta 2-Glycoprotein I: a plasma inhibitor of the contact activation of the intrinsic blood coagulation pathway". Blood 66 (5): 1086–91. doi:10.1182/blood.V66.5.1086.1086. PMID 4052628.
- ↑ "Prothrombinase activity of human platelets is inhibited by beta 2-glycoprotein-I". Biochim. Biophys. Acta 884 (1): 142–9. 1986. doi:10.1016/0304-4165(86)90237-0. PMID 3768409.
- ↑ "Anticardiolipin antibodies block the inhibition by beta 2-glycoprotein I of the factor Xa generating activity of platelets". Thromb. Haemost. 70 (2): 342–5. 1993. doi:10.1055/s-0038-1649577. PMID 8236146.
- ↑ "Synchronized inhibition of the phospholipid mediated autoactivation of factor XII in plasma by beta 2-glycoprotein I and anti-beta 2-glycoprotein I". Thromb. Haemost. 73 (5): 798–804. 1995. doi:10.1055/s-0038-1653871. PMID 7482406.
- ↑ "Role of beta 2-glycoprotein I and anti-phospholipid antibodies in activation of protein C in vitro". J. Clin. Pathol. 46 (10): 908–11. 1993. doi:10.1136/jcp.46.10.908. PMID 8227406.
- ↑ "Inhibitory activity of anti-beta 2-glycoprotein I antibody on factor Va degradation by activated-protein C and its cofactor protein S". Am. J. Hematol. 49 (1): 89–91. 1995. doi:10.1002/ajh.2830490116. PMID 7741146.
- ↑ "beta 2-Glycoprotein I modulates the anticoagulant activity of activated protein C on the phospholipid surface". Thromb. Haemost. 75 (1): 49–55. 1996. doi:10.1055/s-0038-1650220. PMID 8713779.
- ↑ "Beta2-glycoprotein I dependent anticardiolipin antibodies and lupus anticoagulant in patients with recurrent pregnancy loss". Journal of Postgraduate Medicine 48 (1): 5–10. 2002. PMID 12082318.
- ↑ "Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H)". Proc. Natl. Acad. Sci. U.S.A. 87 (11): 4120–4. 1990. doi:10.1073/pnas.87.11.4120. PMID 2349221. Bibcode: 1990PNAS...87.4120M.
- ↑ "A phospholipid-beta 2-glycoprotein I complex is an antigen for anticardiolipin antibodies occurring in autoimmune disease but not with infection". Lupus 1 (2): 75–81. 1992. doi:10.1177/096120339200100204. PMID 1301967.
- ↑ 17.0 17.1 17.2 "Domain V of beta2-glycoprotein I binds factor XI/XIa and is cleaved at Lys317-Thr318.". J Biol Chem 280 (2): 907–12. 2005. doi:10.1074/jbc.M410291200. PMID 15522884.
- ↑ 18.0 18.1 "Identification of the phospholipid-binding site of human beta(2)-glycoprotein I domain V by heteronuclear magnetic resonance". J. Mol. Biol. 304 (5): 927–39. December 2000. doi:10.1006/jmbi.2000.4243. PMID 11124037.
External links
- Apolipoprotein+H at the US National Library of Medicine Medical Subject Headings (MeSH)
- Apolipoprotein H and Applied Research
- Human APOH genome location and APOH gene details page in the UCSC Genome Browser.
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Apolipoprotein H (B2G1)
 |

