Chemistry:Mercury sulfide: Difference between revisions
Pchauhan2001 (talk | contribs) (simplify) |
(link) |
||
| Line 1: | Line 1: | ||
{{About|the mercuric salt|the hypothesized mercurous salt|mercury(I) sulfide}} | |||
{{Chembox | {{Chembox | ||
| Verifiedfields = changed | | Verifiedfields = changed | ||
| Line 15: | Line 16: | ||
| CASNo = 1344-48-5 | | CASNo = 1344-48-5 | ||
| CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| ChemSpiderID = 8395759 | |||
| EC_number = 215-696-3 | | EC_number = 215-696-3 | ||
| UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| Line 45: | Line 47: | ||
| ExternalSDS = [https://www.fishersci.com/store/msds?partNumber=AA1348322&productDescription=MERCURY%28II%29+SULFIDE+BLACK+100G&vendorId=VN00024248&countryCode=US&language=en Fisher Scientific] | | ExternalSDS = [https://www.fishersci.com/store/msds?partNumber=AA1348322&productDescription=MERCURY%28II%29+SULFIDE+BLACK+100G&vendorId=VN00024248&countryCode=US&language=en Fisher Scientific] | ||
| GHSPictograms = {{GHS skull and crossbones}}{{GHS exclamation mark}}{{GHS health hazard}}{{GHS environment}} | | GHSPictograms = {{GHS skull and crossbones}}{{GHS exclamation mark}}{{GHS health hazard}}{{GHS environment}} | ||
| GHSSignalWord = | | GHSSignalWord = Danger | ||
| HPhrases = {{H-phrases|300|310|317|330|373|410}} | | HPhrases = {{H-phrases|300|310|317|330|373|410}} | ||
| PPhrases = {{P-phrases|261|272|280|302+352|321|333+313|363|501}} | | PPhrases = {{P-phrases|261|272|280|302+352|321|333+313|363|501}} | ||
| Line 67: | Line 69: | ||
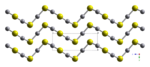
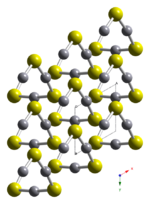
[[File:HgS-alpha-cinnabar-xtal-1999-looking-down-c-axis-CM-3D-balls.png|left|thumb|Structure of a-HgS looking at the c-axis|150px]] | [[File:HgS-alpha-cinnabar-xtal-1999-looking-down-c-axis-CM-3D-balls.png|left|thumb|Structure of a-HgS looking at the c-axis|150px]] | ||
HgS is dimorphic with two crystal forms: | HgS is dimorphic with two crystal forms: | ||
* red [[Chemistry:Cinnabar|cinnabar]] (α-HgS, [[Physics:Trigonal crystal system|trigonal]], hP6, P3221) is the form in which mercury is most commonly found in nature. Cinnabar has rhombohedral crystal system. Crystals of red are optically active. This is caused by the Hg-S helices in the structure.<ref>{{cite journal |author=A. M. Glazer, K. Stadnicka |year=1986 |title=On the origin of optical activity in crystal structures |journal=J. Appl. Crystallogr. |volume=19 |pages=108–122 |doi=10.1107/S0021889886089823 |issue=2 |s2cid=96545158}}</ref> | * red [[Chemistry:Cinnabar|cinnabar]] (α-HgS, [[Physics:Trigonal crystal system|trigonal]], hP6, P3221) is the form in which mercury is most commonly found in nature. Cinnabar has rhombohedral crystal system. Crystals of red are optically active. This is caused by the Hg-S helices in the structure.<ref>{{cite journal |author=A. M. Glazer, K. Stadnicka |year=1986 |title=On the origin of optical activity in crystal structures |journal=J. Appl. Crystallogr. |volume=19 |pages=108–122 |doi=10.1107/S0021889886089823 |issue=2 |bibcode=1986JApCr..19..108G |s2cid=96545158}}</ref> | ||
* black [[Chemistry:Metacinnabar|metacinnabar]] (β-HgS) is less common in nature and adopts the [[Chemistry:Zincblende (crystal structure)|zinc blende crystal structure]] (''T''<sup>2</sup><sub>d</sub>-''F''{{overline|4}}'' | * black [[Chemistry:Metacinnabar|metacinnabar]] (β-HgS) is less common in nature and adopts the [[Chemistry:Zincblende (crystal structure)|zinc blende crystal structure]] (''T''<sup>2</sup><sub>d</sub>-''F''{{overline|4}}3''m''). | ||
==Preparation and chemistry== | ==Preparation and chemistry== | ||
| Line 77: | Line 79: | ||
==Uses== | ==Uses== | ||
[[File: | [[File:Cinnabarit 01.jpg|thumb|left|[[Chemistry:Cinnabar|Cinnabar]] (red portion of specimen)]] | ||
When α-HgS is used as a red pigment, it is known as [[Chemistry: | When α-HgS is used as a red pigment, it is known as [[Chemistry:Cinnabar|cinnabar]]. The tendency of cinnabar to darken has been ascribed to conversion from red α-HgS to black β-HgS. However β-HgS was not detected at excavations in Pompeii, where originally red walls darkened, and was attributed to the formation of Hg-Cl compounds (e.g., [[Chemistry:Corderoite|corderoite]], [[Chemistry:Calomel|calomel]], and [[Chemistry:Terlinguaite|terlinguaite]]) and [[Chemistry:Calcium sulfate|calcium sulfate]], gypsum.<ref> {{cite journal |last1=Cotte |first1=M |author2=Susini J |author3=Metrich N |author4=Moscato A |author5=Gratziu C |author6=Bertagnini A |author7=Pagano M |year=2006 |title=Blackening of Pompeian Cinnabar Paintings: X-ray Microspectroscopy Analysis |journal=Anal. Chem. |volume=78 |issue=21 |pages=7484–7492 |doi=10.1021/ac0612224 |pmid=17073416}}</ref> | ||
As the mercury cell as used in the chlor-alkali industry ([[Physics:Castner–Kellner process|Castner–Kellner process]]) is being phased out over concerns over mercury emissions, the metallic mercury from these setups is converted into mercury sulfide for underground storage. | As the mercury cell as used in the chlor-alkali industry ([[Physics:Castner–Kellner process|Castner–Kellner process]]) is being phased out over concerns over mercury emissions, the metallic mercury from these setups is converted into mercury sulfide for underground storage. | ||
With a band gap of 2.1 eV and its stability, it is possible to be used as [[Chemistry:Photoelectrochemical cell|photoelectrochemical cell]]<ref>{{Cite journal |last1=Davidson |first1=R. S. |last2=Willsher |first2=C. J. |date=March 1979 |title=Mercury(II) sulphide: a photo-stable semiconductor |url=https://www.nature.com/articles/278238a0 |journal=Nature |language=en |volume=278 |issue=5701 |pages=238–239 |doi=10.1038/278238a0 |s2cid=4363745 |issn=1476-4687}}</ref> | With a band gap of 2.1 eV and its stability, it is possible to be used as [[Chemistry:Photoelectrochemical cell|photoelectrochemical cell]].<ref>{{Cite journal |last1=Davidson |first1=R. S. |last2=Willsher |first2=C. J. |date=March 1979 |title=Mercury(II) sulphide: a photo-stable semiconductor |url=https://www.nature.com/articles/278238a0 |journal=Nature |language=en |volume=278 |issue=5701 |pages=238–239 |doi=10.1038/278238a0 |bibcode=1979Natur.278..238D |s2cid=4363745 |issn=1476-4687|url-access=subscription }}</ref> | ||
Neutralization with sulfur has been suggested to clean mercury spills, but the reaction does not proceed rapidly and completely enough for emergencies.<ref>{{cite web|url=https://labphoto.tumblr.com/post/105712717764/how-not-to-clean-up-mercury-at-us-in-secondary|title=How NOT to clean up mercury....|date=20 Dec 2014|first=Kristof|last=Hegedüs|publisher=[[Company:Tumblr|Tumblr]]|access-date=4 January 2025|archive-url=https://web.archive.org/web/20201111192851/https://labphoto.tumblr.com/post/105712717764/how-not-to-clean-up-mercury-at-us-in-secondary|archive-date=11 November 2020|website=Pictures from an Organic Chemistry Laboratory|url-status=live}}</ref> | |||
==See also== | ==See also== | ||
Latest revision as of 19:01, 23 July 2025

| |
| Names | |
|---|---|
| IUPAC name
Mercury sulfide
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2025 |
| |
| |
| Properties | |
| HgS | |
| Molar mass | 232.66 g/mol |
| Density | 8.10 g/cm3 |
| Melting point | 580 °C (1,076 °F; 853 K) decomposes |
| insoluble | |
| Band gap | 2.1 eV (direct, α-HgS) [1] |
| −55.4·10−6 cm3/mol | |
Refractive index (nD)
|
w=2.905, e=3.256, bire=0.3510 (α-HgS) [2] |
| Thermochemistry | |
Std molar
entropy (S |
78 J·mol−1·K−1[3] |
Std enthalpy of
formation (ΔfH⦵298) |
−58 kJ·mol−1[3] |
| Hazards | |
| Safety data sheet | Fisher Scientific |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H300, H310, H317, H330, H373, H410 | |
| P261, P272, P280, P302+352, P321, P333+313, P363, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Mercury oxide mercury selenide mercury telluride |
Other cations
|
Zinc sulfide cadmium sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mercury sulfide, or mercury(II) sulfide is a chemical compound composed of the chemical elements mercury and sulfur. It is represented by the chemical formula HgS. It is virtually insoluble in water.[4]
Crystal structure
HgS is dimorphic with two crystal forms:
- red cinnabar (α-HgS, trigonal, hP6, P3221) is the form in which mercury is most commonly found in nature. Cinnabar has rhombohedral crystal system. Crystals of red are optically active. This is caused by the Hg-S helices in the structure.[5]
- black metacinnabar (β-HgS) is less common in nature and adopts the zinc blende crystal structure (T2d-F43m).
Preparation and chemistry
β-HgS precipitates as a black solid when Hg(II) salts are treated with H2S. The reaction is conveniently conducted with an acetic acid solution of mercury(II) acetate. With gentle heating of the slurry, the black polymorph converts to the red form.[6] β-HgS is unreactive to all but concentrated acids.[4]
Mercury is produced from the cinnabar ore by roasting in air and condensing the vapour.[4]
- HgS → Hg + S
Uses

When α-HgS is used as a red pigment, it is known as cinnabar. The tendency of cinnabar to darken has been ascribed to conversion from red α-HgS to black β-HgS. However β-HgS was not detected at excavations in Pompeii, where originally red walls darkened, and was attributed to the formation of Hg-Cl compounds (e.g., corderoite, calomel, and terlinguaite) and calcium sulfate, gypsum.[7]
As the mercury cell as used in the chlor-alkali industry (Castner–Kellner process) is being phased out over concerns over mercury emissions, the metallic mercury from these setups is converted into mercury sulfide for underground storage.
With a band gap of 2.1 eV and its stability, it is possible to be used as photoelectrochemical cell.[8]
Neutralization with sulfur has been suggested to clean mercury spills, but the reaction does not proceed rapidly and completely enough for emergencies.[9]
See also
- Mercury poisoning
- Mercury(I) sulfide (mercurous sulfide, Hg2S), hypothetical
References
- ↑ L. I. Berger, Semiconductor Materials (1997) CRC Press ISBN:0-8493-8912-7
- ↑ Webminerals
- ↑ 3.0 3.1 Zumdahl, Steven S. (2009). Chemical Principles 6th Ed.. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ↑ 4.0 4.1 4.2 Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 1406. ISBN 978-0-08-022057-4. https://books.google.com/books?id=OezvAAAAMAAJ&q=0-08-022057-6&dq=0-08-022057-6&source=bl&ots=m4tIRxdwSk&sig=XQTTjw5EN9n5z62JB3d0vaUEn0Y&hl=en&sa=X&ei=UoAWUN7-EM6ziQfyxIDoCQ&ved=0CD8Q6AEwBA.
- ↑ A. M. Glazer, K. Stadnicka (1986). "On the origin of optical activity in crystal structures". J. Appl. Crystallogr. 19 (2): 108–122. doi:10.1107/S0021889886089823. Bibcode: 1986JApCr..19..108G.
- ↑ Newell, Lyman C.; Maxson, R. N.; Filson, M. H. (1939). "Red Mercuric Sulfide". Inorganic Syntheses. 1. pp. 19–20. doi:10.1002/9780470132326.ch7. ISBN 9780470132326.
- ↑ Cotte, M; Susini J; Metrich N; Moscato A; Gratziu C; Bertagnini A; Pagano M (2006). "Blackening of Pompeian Cinnabar Paintings: X-ray Microspectroscopy Analysis". Anal. Chem. 78 (21): 7484–7492. doi:10.1021/ac0612224. PMID 17073416.
- ↑ Davidson, R. S.; Willsher, C. J. (March 1979). "Mercury(II) sulphide: a photo-stable semiconductor" (in en). Nature 278 (5701): 238–239. doi:10.1038/278238a0. ISSN 1476-4687. Bibcode: 1979Natur.278..238D. https://www.nature.com/articles/278238a0.
- ↑ Hegedüs, Kristof (20 Dec 2014). "How NOT to clean up mercury....". Tumblr. https://labphoto.tumblr.com/post/105712717764/how-not-to-clean-up-mercury-at-us-in-secondary.
 |