Chemistry:Ensitrelvir
 | |
| Clinical data | |
|---|---|
| Trade names | Xocova |
| Other names | S-217622 |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
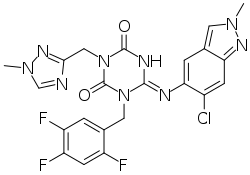
| Formula | C22H17ClF3N9O2 |
| Molar mass | 531.88 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ensitrelvir, sold under the brand name Xocova is an antiviral medication used as a treatment for COVID-19.[2][3][4][5] It was developed by Shionogi in partnership with Hokkaido University and acts as an orally active 3C-like protease inhibitor.[6][7] It is taken by mouth.[1][8][9]
The most common adverse events include transient decreases in high-density lipoprotein and increases blood triglycerides.[1]
Medical uses
Ensitrelvir is indicated for the treatment of COVID-19.[1]
History
(As of 2022), ensitrelvir had reached Phase III clinical trials.[10] The Japanese government is reportedly considering allowing Shionogi permission to apply for approval for medical use before the final steps of trials are completed, potentially speeding up the release for sale. This conditional early approval system has previously been used in Japan to accelerate the progression to market of other antiviral drugs targeting COVID-19, including remdesivir and molnupiravir.[11] In a study of 428 patients, viral load was reduced, but symptoms were not significantly reduced.[12]
In February 2022, the company sought emergency approval from regulators in Japan.[3][12]
Shionogi announced they had reached a preliminary agreement to supply 1 million doses to the Japanese government once the drug is approved. The CEO said they could have capacity to make 10 million doses a year.[13]
Ensitrelvir may be effective in treating smell and taste loss from COVID-19 infection. In a 2023 study, the drug was associated with a 39% reduction in these symptoms.[14]
Society and culture
Legal status
Ensitrelvir was approved for emergency use in Japan in November 2022.[1][3][4]
Names
Ensitrelvir is the International Nonproprietary Name.[15]
Research
Ensitrelvir is being studied for its potential use as post-exposure prophylaxis (PEP) after SARS-CoV-2 exposure.[16][17] The SCORPIO-PEP trial is a global Phase 3 trial that will evaluate the safety and efficacy of the drug in preventing symptomatic SARS-CoV-2 infection in household contacts of people who tested positive for COVID-19.[17][18][19]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Xocova (Ensitrelvir Fumaric Acid) Tablets 125mg Approved in Japan for the Treatment of SARS-CoV-2 Infection, under the Emergency Regulatory Approval System". Shionogi (Press release). 22 November 2022. Retrieved 28 November 2022.
- ↑ "Ensitrelvir as a potential treatment for COVID-19". Expert Opin Pharmacother 23 (18): 1995–1998. December 2022. doi:10.1080/14656566.2022.2146493. PMID 36350029.
- ↑ 3.0 3.1 3.2 Fujikawa, Megumi (22 November 2022). "Japan Approves First Homegrown Covid-19 Antiviral Pill". https://www.wsj.com/articles/japan-approves-first-homegrown-covid-19-antiviral-pill-11669117019.
- ↑ 4.0 4.1 "Shionogi's Covid antiviral lands first approval in Japan's new emergency approval pathway". 22 November 2022. https://endpts.com/shionogis-covid-antiviral-lands-first-approval-in-japans-new-emergency-approval-pathway/.
- ↑ "Xocova: Powerful New Japanese Pill for Coronavirus Treatment.". BioPharma Media. February 2022. https://biopharma.media/xocova-powerful-new-japanese-drug-for-coronavirus-treatment-3494/.
- ↑ "Discovery of S-217622, a Noncovalent Oral SARS-CoV-2 3CL Protease Inhibitor Clinical Candidate for Treating COVID-19". Journal of Medicinal Chemistry 65 (9): 6499–6512. May 2022. doi:10.1021/acs.jmedchem.2c00117. PMID 35352927.
- ↑ "Shionogi presents positive Ph II/III results for COVID-19 antiviral S-217622". thepharmaletter.com. 31 January 2022. https://www.thepharmaletter.com/article/shionogi-presents-positive-ph-ii-iii-results-for-covid-19-antiviral-s-217622.
- ↑ "Shionogi's new COVID pill appears to ease omicron symptoms.". Nikkei Asia. 21 December 2021. https://asia.nikkei.com/Spotlight/Coronavirus/Shionogi-s-new-COVID-pill-appears-to-ease-omicron-symptoms.
- ↑ "Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA.2". Nature 607 (7917): 119–127. May 2022. doi:10.1038/s41586-022-04856-1. PMID 35576972. Bibcode: 2022Natur.607..119U.
- ↑ "S-217622, a 3CL Protease Inhibitor and Clinical Candidate for SARS-CoV-2". Journal of Medicinal Chemistry 65 (9): 6496–6498. May 2022. doi:10.1021/acs.jmedchem.2c00624. PMID 35507419.
- ↑ "Japan to consider early approval for Shionogi COVID-19 pill.". Japan Times. 8 February 2022. https://www.japantimes.co.jp/news/2022/02/08/national/shionogi-covid-drug-early-approval/.
- ↑ 12.0 12.1 "Japan's Shionogi seeks approval for COVID-19 pill". Reuters. 25 February 2022. https://www.reuters.com/business/healthcare-pharmaceuticals/japans-shionogi-seeks-approval-oral-covid-19-drug-2022-02-25/.
- ↑ "Japan's Shionogi signs government supply pact for pill to fight COVID". Reuters. 25 March 2022. https://www.reuters.com/business/healthcare-pharmaceuticals/japans-shionogi-signs-govt-supply-pact-oral-covid-drug-2022-03-25/.
- ↑ Lenharo, Mariana (2023-10-17). "New pill helps COVID smell and taste loss fade quickly" (in en). Nature. doi:10.1038/d41586-023-03244-7. PMID 37853192. https://www.nature.com/articles/d41586-023-03244-7.
- ↑ "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 88". WHO Drug Information 36 (3): 89. 2022.
- ↑ Cosdon, Nina (31 March 2023). "Ensitrelvir: A COVID-19 Antiviral That Remains Effective Against New Variants". https://www.contagionlive.com/view/ensitrelvir-a-covid-19-antiviral-that-remains-effective-against-new-variants.
- ↑ 17.0 17.1 "Shionogi presses on with Xocova research following Japanese approval". 16 February 2023. https://www.thepharmaletter.com/article/shionogi-presses-on-with-xocova-research-following-japanese-approval.
- ↑ "Studies Currently Enrolling". https://www.kumc.edu/school-of-medicine/campuses/wichita/research/center-for-clinical-research-wichita/clinical-trials/studies-currently-enrolling.html. "SCORPIO-PEP is a 28-day study to assess the prevention of COVID-19 infection in those who have been exposed through household contact."
- ↑ "Shionogi Enrolls the First Participant in Japan in its Global Phase 3 Trial of Ensitrelvir for the Prevention of Symptomatic SARS-CoV-2 Infection" (Press release). Osaka, Japan. 9 June 2023. Retrieved 28 October 2023.
External links
- "Ensitrelvir". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/ensitrelvir.
 |


