Chemistry:Permanganic acid
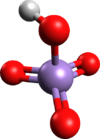
| |
| Names | |
|---|---|
| IUPAC name
Permanganic acid
| |
| Other names
Manganic(VII) acid
Hydroxy(trioxo)manganese Hydrogen permanganate | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| HMnO4 | |
| Molar mass | 119.94 g mol−1 |
| Appearance | Violet |
| Acidity (pKa) | about -4.6 to -2.3 [1][2] |
| Conjugate base | Permanganate |
| Hazards | |
| Main hazards | Oxidant, Corrosive |
| Related compounds | |
Other cations
|
Potassium permanganate Sodium permanganate Calcium permanganate |
Related compounds
|
Pertechnetic acid Perrhenic acid Perchloric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Permanganic acid (or manganic(VII) acid) is the inorganic compound with the formula HMnO4. This strong oxoacid has been isolated as its dihydrate. It is the conjugate acid of permanganate salts. It is the subject of few publications and its characterization as well as its uses are very limited.
Preparation and structure
Permanganic acid is most often prepared by the reaction of dilute sulfuric acid with a solution of barium permanganate, the insoluble barium sulfate byproduct being removed by filtering:[3]
- Ba(MnO4)2 + H2SO4 → 2 HMnO4 + BaSO4↓
The sulfuric acid used must be dilute; reactions of permanganates with concentrated sulfuric acid yield the anhydride, manganese heptoxide.
Permanganic acid has also been prepared through the reaction of hydrofluorosilicic acid with potassium permanganate,[4] through electrolysis, and through hydrolysis of manganese heptoxide, though the last route often results in explosions.[5]
Crystalline permanganic acid has been prepared at low temperatures as the dihydrate, HMnO4·2H2O.[3]
Although its structure has not been verified spectroscopically or crystallographically, HMnO4 is assumed to be adopt a tetrahedral structure akin to that for perchloric acid.
Reactions
As a strong acid, HMnO4 is deprotonated to form the intensely purple coloured permanganates. Potassium permanganate, KMnO4, is a widely used, versatile and powerful oxidising agent.
Permanganic acid solutions are unstable, and gradually decompose into manganese dioxide, oxygen, and water, with initially formed manganese dioxide catalyzing further decomposition.[6] Decomposition is accelerated by heat, light, and acids. Concentrated solutions decompose more rapidly than dilute.[6]
References
- ↑ Stewart, R.; Mocek, M.M. (1963). "The Mechanisms of Permanganate Oxidation VII: The Oxidation of Fluoral Hydrate". Canadian Journal of Chemistry 41 (5): 1160–9. doi:10.1139/v63-164.
- ↑ Bailey, N.; Carrington, A.; Lott, K.A.K.; Symons, M.C.R (1960). "Structure and Reactivity of the Oxyanions of Transition Metals Part VII: Acidities and Spectra of Protonated Oxyanions". Journal of the Chemical Society (Resumed): 290–7. doi:10.1039/JR9600000290.
- ↑ 3.0 3.1 Frigerio, Norman A. (1969). "Preparation and properties of crystalline permanganic acid". Journal of the American Chemical Society 91 (22): 6200–1. doi:10.1021/ja01050a058. PMID 5823192.
- ↑ Black, Homer Van Valkenburg (1900). The permanganates of barium, strontium, and calcium.. Easton, PA. p. 6. https://books.google.com/books?id=HZNPAAAAYAAJ.
- ↑ Olsen, J. C. (1900). Permanganic Acid by Electrolysys. Easton, PA: The Chemical Publishing Company. https://books.google.com/books?id=EJNPAAAAYAAJ.
- ↑ 6.0 6.1 Byers, Horace Greeley (1899). A Study of the Reduction of Permanganic acid by Manganese Dioxide. Chemical Publishing Company. https://archive.org/details/studyofreduction00byer.
 |

