Chemistry:Bismuth subsalicylate
 | |
| Clinical data | |
|---|---|
| Trade names | Pepto-Bismol, BisBacter |
| AHFS/Drugs.com | Multum Consumer Information |
| MedlinePlus | a607040 |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C7H5BiO4 |
| Molar mass | 362.093 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bismuth subsalicylate, sold generically as pink bismuth and under brand names including Pepto-Bismol, Pepti-Calm and BisBacter, is a medication used to treat temporary discomfort of the stomach and gastrointestinal tract, such as nausea, heartburn, indigestion, upset stomach, and diarrhea.
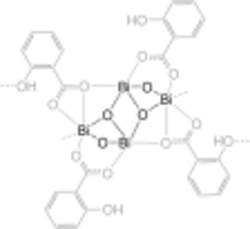
Bismuth subsalicylate has the empirical chemical formula C7H5BiO4,[1] and is a colloidal substance obtained by hydrolysis of bismuth salicylate (Bi(C6H4(OH)CO2)3).
Medical uses
As a derivative of salicylic acid, bismuth subsalicylate displays anti-inflammatory[2] and bactericidal action.[3] It also acts as an antacid.
Mechanism of action
Bismuth subsalicylate is used as an antacid and antidiarrheal, and to treat some other gastrointestinal symptoms, such as nausea. The means by which this occurs is still not well documented. It is thought to be some combination of the following:[4]
- Stimulation of absorption of fluids and electrolytes by the intestinal wall (antisecretory action)
- As a salicylate, reducing inflammation/irritation of stomach and intestinal lining through inhibition of prostaglandin G/H synthase 1/2
- Reduction in hypermotility of the stomach
- Inhibits adhesion and filmogenesis by Escherichia coli
- Bactericidal action of a number of its subcomponents, including salicylic acid[5]
- Bactericidal action via a so-called oligodynamic effect in which small amounts of heavy metals such as bismuth damage many different bacteria species.
- Weak antacid properties
In vitro and in vivo data have shown that bismuth subsalicylate hydrolyzes in the gut to bismuth oxychloride and salicylic acid and less commonly bismuth hydroxide. In the stomach, this is likely an acid-catalyzed hydrolysis. The salicylic acid is absorbed and therapeutical concentrations of salicylic acid can be found in blood after bismuth subsalicylate administration. Bismuth oxychloride and bismuth hydroxide are both believed to have bactericidal effects, as is salicylic acid for enterotoxigenic E. coli a common cause of "traveler's diarrhea."[5]
Organobismuth compounds have historically been used in growth media for selective isolation of microorganisms. Such salts have been shown to inhibit proliferation of Helicobacter pylori, other enteric bacteria, and some fungi.[6]
Adverse effects
There are some adverse effects. It can cause a black tongue and black stools in some users of the drug when it combines with trace amounts of sulfur in saliva and the colon to form bismuth sulfide.[7] Bismuth sulfide is a highly insoluble black salt, and the discoloration seen is temporary and harmless.
Long-term use (more than six weeks) may lead to accumulation and toxicity.[8] Some of the risks of salicylism can apply to the use of bismuth subsalicylate.[9][10][11]
Children should not take medication with bismuth subsalicylate while recovering from influenza or chicken pox, as epidemiologic evidence points to an association between the use of salicylate-containing medications during certain viral infections and the onset of Reye syndrome.[12] For the same reason, it is typically recommended that nursing mothers not use medication containing bismuth subsalicylate because small amounts of the medication are excreted in human breast milk, and these pose a theoretical risk of Reye's syndrome to nursing children.[13]
Salicylates are very toxic to cats, and thus bismuth subsalicylate should not be administered to cats.[14]
The British National Formulary does not recommend bismuth-containing antacids (unless chelated), cautioning that absorbed bismuth can be neurotoxic, causing encephalopathy, and that such antacids tend to be constipating.[15]
Drug interactions
There is an increased risk of bleeding when using bismuth subsalicylate and anticoagulation therapy, like Coumadin (Warfarin).[16][17][18]
History
Bismuth salts were in use in Europe by the late 1700s. The combination of bismuth subsalicylate and zinc salts for astringency with salol (phenyl salicylate) appears to have begun in the US in the early 20th century as a remedy for life-threatening diarrhea in infants with cholera. At first sold directly to physicians, it was first marketed as Bismosal in 1918.[19]
Pepto-Bismol was first sold in 1900[19] or 1901[20] by a doctor in New York. It was originally sold as a remedy for infant diarrhea by Norwich Pharmacal Company under the name "Bismosal: Mixture Cholera Infantum".[19] It was renamed Pepto-Bismol in 1919. Norwich Eaton Pharmaceuticals was acquired by Procter and Gamble in 1982.[21]
As of 1946 and 1959, Canadian advertisements placed by Norwich show the product as Pepto-Besmal both in graphic and text.[22][23]
Pepto-Bismol is an over-the-counter drug currently produced by the Procter & Gamble company in the United States, Canada and the United Kingdom. Pepto-Bismol is made in chewable tablets[24] and swallowable caplets,[25] but it is best known for its original formula, which is a thick liquid. This original formula is a medium pink in color, with a teaberry (methyl salicylate) flavor.[26]
Generic bismuth subsalicylate and other branded versions of the drug are widely available in pill and liquid form.
Structure

Despite its common usage and commercial significance, the exact structure of the pharmaceutical long remained undetermined, but was revealed, through the use of advanced electron crystallography techniques, to be a layered coordination polymer with the formula BiO(C7H5O3).[27] In the structure, both the carboxylate and phenol groups of the salicylate coordinate towards the bismuth cations. The determination of bismuth subsalicylate had long been hindered due to the small particle size as well as defects within the structure, arising from variations in the stacking arrangement of the bismuth subsalicylate layers, which could be observed as part of the structural investigation.[28]
References
- ↑ Merck Index, 11th Edition, 1299
- ↑ "Investigational treatment options in microscopic colitis". Expert Opinion on Investigational Drugs 17 (12): 1829–37. December 2008. doi:10.1517/13543780802514500. PMID 19012499.
- ↑ "Travelers' diarrhea: antimicrobial therapy and chemoprevention". Nature Clinical Practice. Gastroenterology & Hepatology 2 (4): 191–8; quiz 1 p following 198. April 2005. doi:10.1038/ncpgasthep0142. PMID 16265184.
- ↑ Bismuth subsalicylate, DrugBank.
- ↑ 5.0 5.1 "Binding and killing of bacteria by bismuth subsalicylate". Antimicrobial Agents and Chemotherapy 33 (12): 2075–82. December 1989. doi:10.1128/AAC.33.12.2075. PMID 2694949.
- ↑ "Metabolism of bismuth subsalicylate and intracellular accumulation of bismuth by Fusarium sp. strain BI". Applied and Environmental Microbiology 71 (2): 876–82. February 2005. doi:10.1128/AEM.71.2.876-882.2005. PMID 15691943. Bibcode: 2005ApEnM..71..876D.
- ↑ "Why does Pepto-Bismol sometimes darken the tongue/stool and how long does it last?". Pepto-Bismol FAQ. Pepto-Bismol. http://www.pepto-bismol.com/en-us/faq/black-stool-black-tongue.
- ↑ "Bismuth therapy in gastrointestinal diseases". Gastroenterology 99 (3): 863–75. September 1990. doi:10.1016/0016-5085(90)90983-8. PMID 2199292.
- ↑ "Bismuth Subsalicylate". MedlinePlus. National Institutes of Health. https://www.nlm.nih.gov/medlineplus/druginfo/medmaster/a607040.html.
- ↑ "Fatal salicylate toxicity from bismuth subsalicylate". The Western Journal of Medicine 155 (6): 637–9. December 1991. PMID 1812638.
- ↑ "Chronic salicylate toxicity due to consumption of over-the-counter bismuth subsalicylate". The American Journal of Medicine 97 (3): 308–9. September 1994. doi:10.1016/0002-9343(94)90017-5. PMID 8092182.
- ↑ Aspirin or Salicylate-Containing Medications, reyessyndrome.org
- ↑ "Food-borne and Waterborne Illness - Breastfeeding – CDC". cdc.gov. https://www.cdc.gov/breastfeeding/disease/food_illness.htm.
- ↑ Cat Owner's Home Veterinary Handbook, Carlson and Giffin, page 390.
- ↑ "1.1.1 Antacids and simeticone". http://services2.ascribe.com:8080/bnf/view/page/bnf/PHP276-antacids-and-simeticone.htm.
- ↑ "Drug Interactions between Pepto-Bismol and warfarin". https://www.drugs.com/drug-interactions/pepto-bismol-with-warfarin-391-177-2311-0.html.
- ↑ International Travel Health Guide 2006-2007. Mosby. 2006. pp. 89–103. ISBN 978-0-323-04050-1.
- ↑ "Inadvertent exaggerated anticoagulation following use of bismuth subsalicylate in an enterally fed patient receiving warfarin therapy". Nutrition in Clinical Practice 28 (6): 766–769. December 2013. doi:10.1177/0884533613507606. PMID 24163322.
- ↑ 19.0 19.1 19.2 "Bismuth subsalicylate: history, chemistry, and safety". Reviews of Infectious Diseases 12 Suppl 1 (Supplement 1): S3-8. January–February 1990. doi:10.1093/clinids/12.supplement_1.s3. PMID 2406853.
- ↑ "History of Pepto Bismol". Procter & Gamble. https://www.pepto-bismol.com/en-us/about/history.
- ↑ Rising Tide: Lessons from 165 Years of Brand Building at Procter and Gamble. Harvard Business Press. May 1, 2004. pp. 424. ISBN 9781591391470. https://books.google.com/books?id=ZyUwNAs43LcC&pg=PA424.
- ↑ "'Simple Diarrhea' ad". Toronto Daily Star: p. 33. August 16, 1946.
- ↑ "'Pepto-Besmal puts out the fire of an upset stomach' ad". Toronto Daily Star. June 6, 1959.
- ↑ http://tess2.uspto.gov/bin/showfield?f=doc&state=b8i462.2.2 The trademark was extended to cover the tablets in 1973. Registration No. 0972198], November 6, 1973.
- ↑ The capsules were introduced in 1983. Registration No. 1269605, March 13, 1984; cancelled July 16, 1990. http://tess2.uspto.gov/bin/showfield?f=doc&state=b8i462.2.1.
- ↑ "Pepto-Bismol Original Liquid". Material Safety Data Sheet. Procter & Gamble. https://www.pg.com/productsafety/msds/health_care/gastrointestinal/Pepto-Bismol_Original_Liquid.pdf.
- ↑ 27.0 27.1 "Structure of the active pharmaceutical ingredient bismuth subsalicylate". Nature Communications 13 (1984): 1984. April 2022. doi:10.1038/s41467-022-29566-0. PMID 35418171.
- ↑ Henry Arnaud, Celia (April 26, 2022). "Structure of Pepto-Bismol active ingredient solved" (in en). Chemical & Engineering News 100 (44): 34–35. doi:10.1021/cen-10044-cover6. ISSN 0009-2347. https://cen.acs.org/materials/inorganic-chemistry/Structure-Pepto-Bismol-active-ingredient/100/web/2022/04. Retrieved 15 April 2023.
External links
- "Towards a structural understanding of the anti-ulcer and anti-gastritis drug bismuth subsalicylate". Angewandte Chemie 45 (34): 5638–42. August 2006. doi:10.1002/anie.200600469. PMID 16865763.
 |



