Chemistry:Riamilovir
 | |
| Clinical data | |
|---|---|
| Other names | TZV, Triazavirin |
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| Chemical and physical data | |
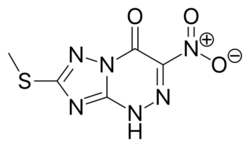
| Formula | C5H4N6O3S |
| Molar mass | 228.19 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Riamilovir is a broad-spectrum antiviral drug developed in Russia through a joint effort of Ural Federal University, Russian Academy of Sciences, Ural Center for Biopharma Technologies and Medsintez Pharmaceutical.[1] It has a novel triazolotriazine core, which represents a new structural class of non-nucleoside antiviral drugs.[2]
The main principle action of triazavirin is to inhibit the synthesis of viral ribonucleic acid (RNA) and the replication of viral genomic fragments through its synthetic analogue to the bases of purine nucleosides.[3][4][5]
Uses
It was originally developed as a potential treatment for pandemic influenza strains such as H5N1, and most of the testing that has been done has focused on its anti-influenza activity.[5][3][6][7][8] However, triazavirin has also been found to have antiviral activity against a number of other viruses including tick-borne encephalitis virus,[4][9] and is also being investigated for potential application against a lethal influenza infection and secondary bacterial pneumonia following influenza,[10] Lassa fever and Ebola virus disease.[11][12][13][14][15] Triazavirin has passed clinical trials and has shown antiviral activity against ARVI.[16][17][18][19] In 2020, testing of triazavirin was started against SARS-CoV-2 in Russia, China, and South Africa. [20][21][22][23][24][25][excessive citations] The mechanism of action of Triazavirin is still controversial. No experimental biochemical studies on the activity of Triazavirin relating to SARS-CoV-2 or influenza target proteins have so far been published.[26]
In August 2014, the Ministry of Health of Russia issued a registration certificate for triazavirin.[27] The active substance of the drug triazavirin is a new active molecule,[2] and can be dispensed by prescription. The production of triazavirin is carried out at a modern pharmaceutical enterprise LLC "Plant Medsintez".[1] The registration procedure for triazavirin has begun in the Republic of South Africa.[24]
Criticism
The studies of Triazavirin were non-blinded and non-randomized, and included 66 patients only with, with 44 in a control group.[16]
References
- ↑ 1.0 1.1 "Triazaverin Is Officially Recommended". https://www.medsintez.com/en/novosti/127-triazavirin-ofitsialno-rekomendovan.
- ↑ 2.0 2.1 "Nucleophilic substitution of nitro group in nitrotriazolotriazines as a model of potential interaction with cysteine-containing proteins.". Chemistry of Heterocyclic Compounds 51 (3): 275–280. 2015. doi:10.1007/s10593-015-1695-4.
- ↑ 3.0 3.1 "Antiviral properties, metabolism, and pharmacokinetics of a novel azolo-1,2,4-triazine-derived inhibitor of influenza A and B virus replication". Antimicrobial Agents and Chemotherapy 54 (5): 2017–2022. May 2010. doi:10.1128/AAC.01186-09. PMID 20194696.
- ↑ 4.0 4.1 "[Investigation of Triazavirin antiviral activity against tick-borne encephalitis pathogen in cell culture]" (in ru). Antibiotiki i Khimioterapiia 59 (1–2): 3–5. 2014. PMID 25051708.
- ↑ 5.0 5.1 "[Investigation of triazavirin antiviral activity against influenza A virus (H5N1) in cell culture]" (in ru). Antibiotiki i Khimioterapiia 52 (11–12): 18–20. 2007. PMID 19275052.
- ↑ "[A new antiviral drug Triazavirin: results of phase II clinical trial]" (in ru). Voprosy Virusologii 57 (6): 9–12. 2012. PMID 23477247.
- ↑ "Nucleoside analogues for the treatment of influenza: History and experience" (in Ru). Journal of Infectology 11 (3): 20–26. 9 October 2019. doi:10.22625/2072-6732-2019-11-3-20-26.
- ↑ "A comparative efficacy and safety of using antiviral drugs in therapy of patients with influenza" (in ru). Infekcionnye Bolezni 15 (3): 25–32. 2017. doi:10.20953/1729-9225-2017-3-25-32.
- ↑ "[Investigation of Therapeutic Efficacy of Triazavirin Against Experimental Forest-Spring Encephalitis on Albino Mice]" (in ru). Antibiotiki i Khimioterapiia 60 (7–8): 11–13. 2015. PMID 26863736.
- ↑ "Effect of triazavirine on the outcome of a lethal influenza infection and secondary bacterial pneumonia following influenza in mice". Microbiology Independent Research Journal 4 (1). 2018-02-22. doi:10.18527/2500-2236-2017-4-1-52-57. https://www.mir-journal.org/issues/4/4/.
- ↑ "Target: Ebola". Pravda. 2014-12-22. http://english.pravda.ru/health/22-12-2014/129359-target_ebola-0/.
- ↑ "Yekaterinburg pharmacies to sell domestic antiviral drug". Yekaterinburg News Reports. 6 January 2015. http://yekaterinburgnews.com/daily-news/yekaterinburg-pharmacies-to-sell-domestic-antiviral-drug/11873/.
- ↑ "Ebola crisis: Vaccine 'too late' for outbreak. BBC News, 17 October 2014". BBC News. 2014-10-17. https://www.bbc.co.uk/news/health-29649572.
- ↑ "Russia Will Begin Testing Triazavirin, Used For Lassa Fever, And Other Drugs On Ebola: Health Ministry.". International Business Times. 12 November 2014. http://www.ibtimes.com/russia-will-begin-testing-triazavirin-used-lassa-fever-other-drugs-ebola-health-1722194.
- ↑ "New antiviral drug from Urals will help fight Ebola and other viruses.". Russia Beyond the Headlines. 12 November 2014. http://rbth.com/science_and_tech/2014/11/12/new_antiviral_drug_from_urals_will_help_fight_ebola_and_othe_41349.html.
- ↑ 16.0 16.1 "Study of effectiveness of antiviral drugs (umifenovir, triazavirin) against acute respiratory viral infections" (in ru). Kazan Medical Journal 99 (2): 215–223. 2018-04-15. doi:10.17816/KMJ2018-215. ISSN 2587-9359. https://kazanmedjournal.ru/kazanmedj/article/view/8409.
- ↑ "The Possibilities of Etiotropic Therapy for Influenza and ARVI with Taking into Account the Period of Hospitalization and the Risk of Developing Secondary Complications". 2019. https://www.antibiotics-chemotherapy.ru/jour/article/view/124.
- ↑ "[The practice of using a domestic antiviral drug in the etiotropic therapy of acute respiratory viral infection]" (in ru). Terapevticheskii Arkhiv 92 (12): 160–164. December 2020. doi:10.26442/00403660.2020.12.200427. PMID 33720589.
- ↑ "Modern Etiotropic Therapy of Influenza and ARVI in Adult Patients with Premorbid Pathology". 2018. https://www.antibiotics-chemotherapy.ru/jour/article/view/96.
- ↑ "China Testing Russia's Triazavirin As Coronavirus Treatment". Russian Health Ministry. Urdupoint. 4 February 2020. https://www.urdupoint.com/en/world/china-testing-russias-triazavirin-as-coronav-828779.html.
- ↑ "China Tests Russian Antiviral Drug Which Might Treat Coronavirus As Moscow Warns Of Possible 'Mass Outbreak'". Zambia Reports. 5 February 2020. https://zambiareports.com/2020/02/05/china-tests-russian-antiviral-drug-might-treat-coronavirus-moscow-warns-possible-mass-outbreak/.
- ↑ "Efficacy and Safety of Triazavirin Therapy for Coronavirus Disease 2019: A Pilot Randomized Controlled Trial". Engineering 6 (10): 1185–1191. October 2020. doi:10.1016/j.eng.2020.08.011. PMID 32923016.
- ↑ "SA study to trial antiviral Triazavirin as COVID-19 treatment" (in en). https://ewn.co.za/2021/01/20/sa-study-to-trial-antiviral-triazavirin-as-covid-19-treatment.
- ↑ 24.0 24.1 "Discussion on Russia's antiviral drug Triazavirin". SABC News. 21 January 2021. https://www.youtube.com/watch?v=qbMpopd7N14&t=22s.
- ↑ "Practical Experience of Using Riamilovir in Treatment of Patients with Moderate COVID-19". Antibiotiki i Khimioterapiya 65 (7–8): 27–30. 21 November 2020. doi:10.37489/0235-2990-2020-65-7-8-27-30.
- ↑ Chupakhin, Oleg N.; Rusinov, Vladimir L.; Varaksin, Mikhail V.; Ulomskiy, Evgeny N.; Savateev, Konstantin V.; Butorin, Ilya I.; Du, Weijie; Sun, Zhiyong et al. (22 November 2022). "Triazavirin—A Novel Effective Antiviral Drug". International Journal of Molecular Sciences 23 (23): 14537. doi:10.3390/ijms232314537. PMID 36498864.
- ↑ "Triazavirin (Riamilovir)" (in Ru). State Register of Medicines of the Russian Federation. http://grls.rosminzdrav.ru/Grls_View_v2.aspx?routingGuid=d552450f-6223-43a2-87af-e5840a632b19&t=.
 |

