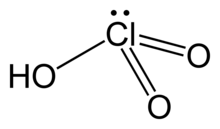
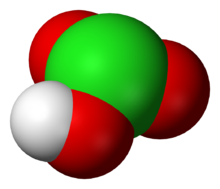
Chemistry:Chloric acid
| |
| |
| Names | |
|---|---|
| Other names
Chloric(V) acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2626 |
| |
| |
| Properties | |
| HClO3 | |
| Molar mass | 84.45914 g mol−1 |
| Appearance | colourless solution |
| Density | 1 g/mL, solution (approximate) |
| >40 g/100 ml (20 °C) | |
| Acidity (pKa) | −2.7[1] |
| Conjugate base | Chlorate |
| Structure | |
| pyramidal | |
| Hazards | |
| Main hazards | Oxidant, Corrosive |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H271, H314 | |
| P210, P220, P221, P260, P264, P280, P283, P301+330+331, P303+361+353, P304+340, P305+351+338, P306+360, P310, P321, P363, P370+378, P371+380+375, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
bromic acid iodic acid |
Other cations
|
ammonium chlorate sodium chlorate potassium chlorate |
Related compounds
|
hydrochloric acid hypochlorous acid chlorous acid perchloric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chloric acid, HClO3, is an oxoacid of chlorine, and the formal precursor of chlorate salts. It is a strong acid (pKa ≈ −2.7) and an oxidizing agent.
Properties
Chloric acid is thermodynamically unstable with respect to disproportionation.
Chloric acid is stable in cold aqueous solution up to a concentration of approximately 30%, and solution of up to 40% can be prepared by careful evaporation under reduced pressure. Above these concentrations, chloric acid solutions decompose to give a variety of products, for example:
- 8 HClO3 → 4 HClO4 + 2 H2O + 2 Cl2 + 3 O2
- 3 HClO3 → HClO4 + H2O + 2 ClO2
Hazards
Chloric acid is a powerful oxidizing agent. Most organics and flammables will deflagrate on contact.
Production
It can be prepared by the reaction of sulfuric acid with barium chlorate, the insoluble barium sulfate being removed by precipitation:
- Ba(ClO3)2 + H2SO4 → 2 HClO3 + BaSO4
Another method is the heating of hypochlorous acid, producing chloric acid and hydrogen chloride:
- 3 HClO → HClO3 + 2 HCl
See also
References
- ↑ Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (2007) (in de). Lehrbuch der anorganischen Chemie. Berlin. ISBN 978-3-11-017770-1. OCLC 180963521.
- R. Bruce King, ed (1994). "Chloric acid". Encyclopedia of Inorganic Chemistry. 2. Chichester: Wiley. p. 658. ISBN 0-471-93620-0.
 |


